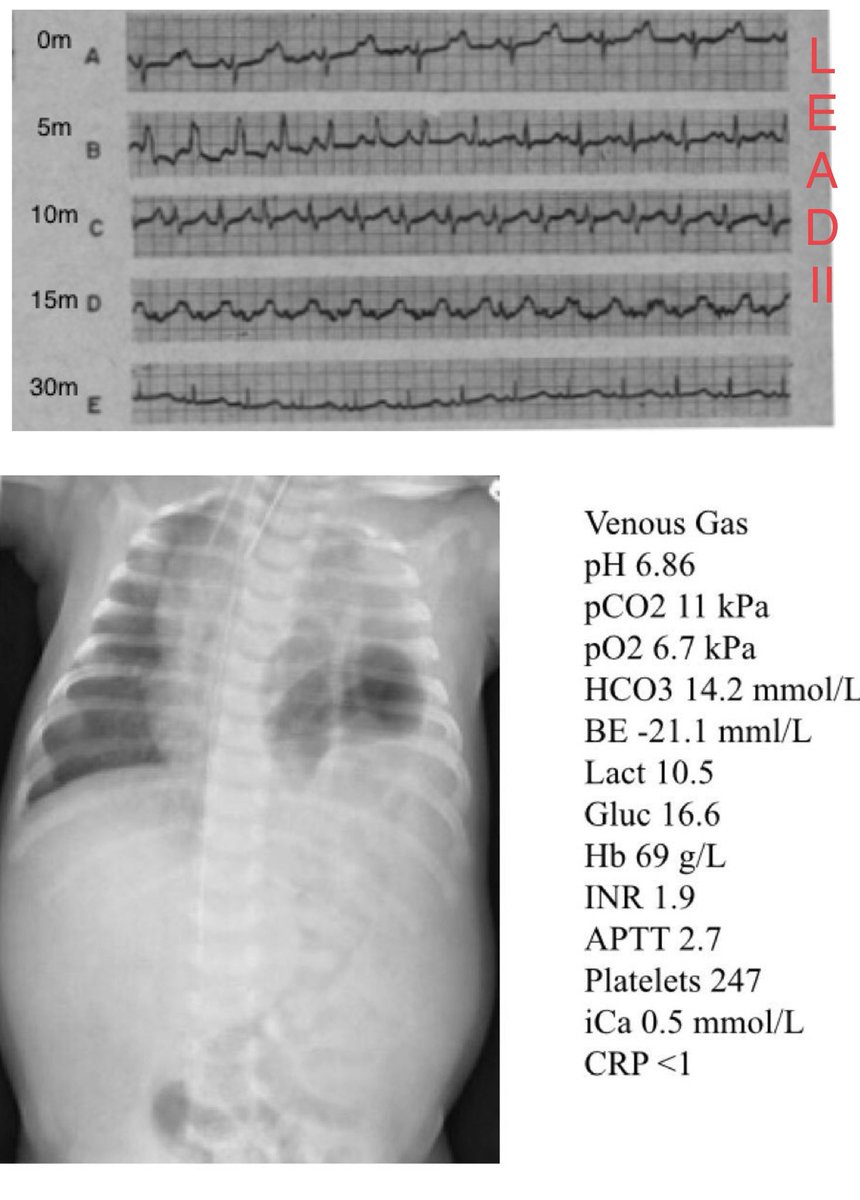
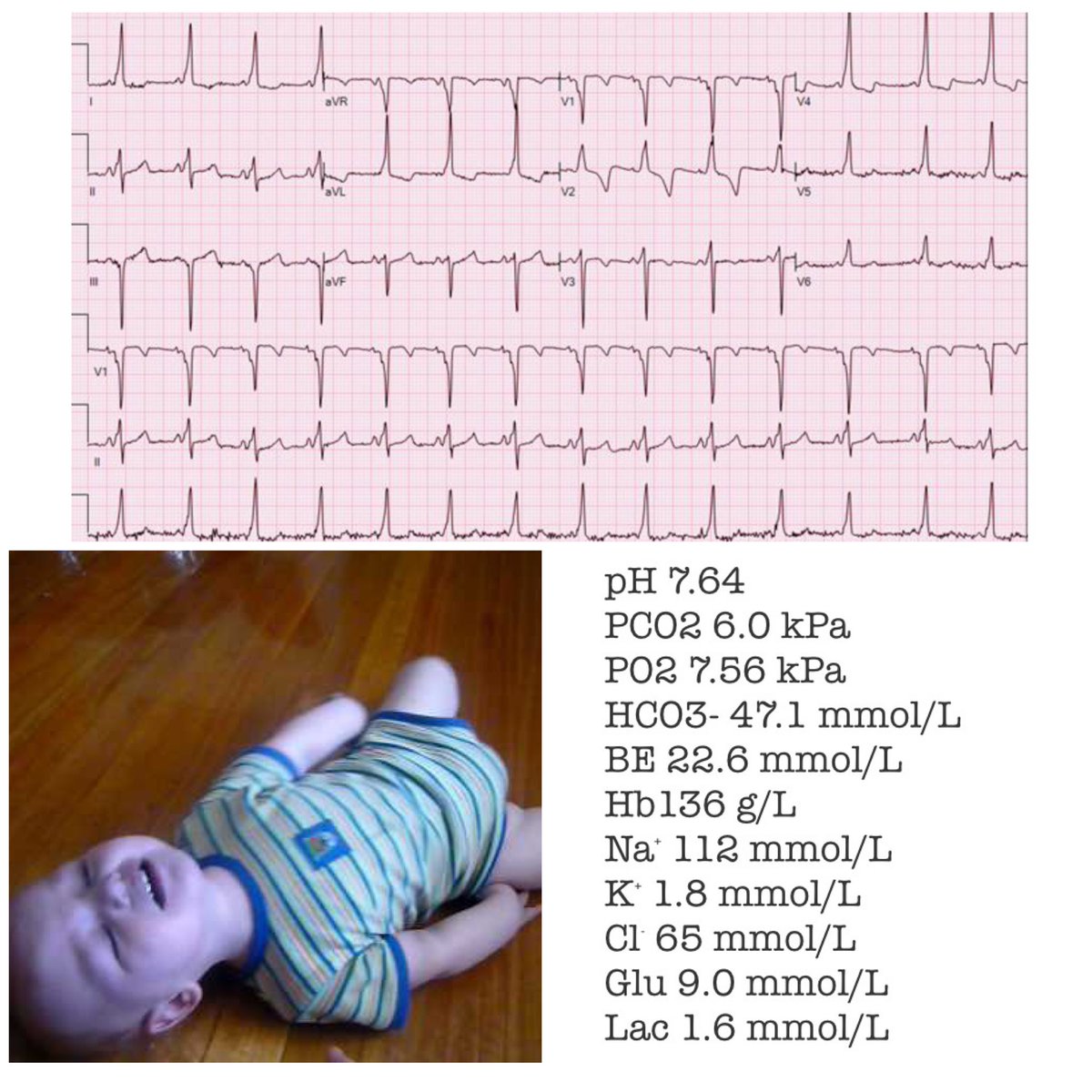
A #pedsicu thread:
How much K+ does Hartmann’s solution and 0.9% Saline solution contain respectively ?
0.9% saline has no K+ content.
So with that in mind, which one do you think is safer to use in a patient with hyperkalaemia (lets assume that's someone with a K+ more ore equal to 5.5mmol/L)
There is a very common misconception that Hartmann’s solution(aka) Ringer’s lactate is contraindicated in patients with hyperkalaemia. We are challenged on this almost weekly when we get pedicu referrals @NWTStransport
The bulk of total body potassium is intracellular (about 98%) with a concentration of roughly 140mmol/L. Even a minute shift from within the cell to the extracellular environment can have a dramatic rise on extracellural K+ concentration. (Ab)normal saline is
If you are interested in the intricacies of K+ homeostasis at a cellular level in response to pH I would highly recommend this paper by Aronson et al bit.ly/2TUUy49
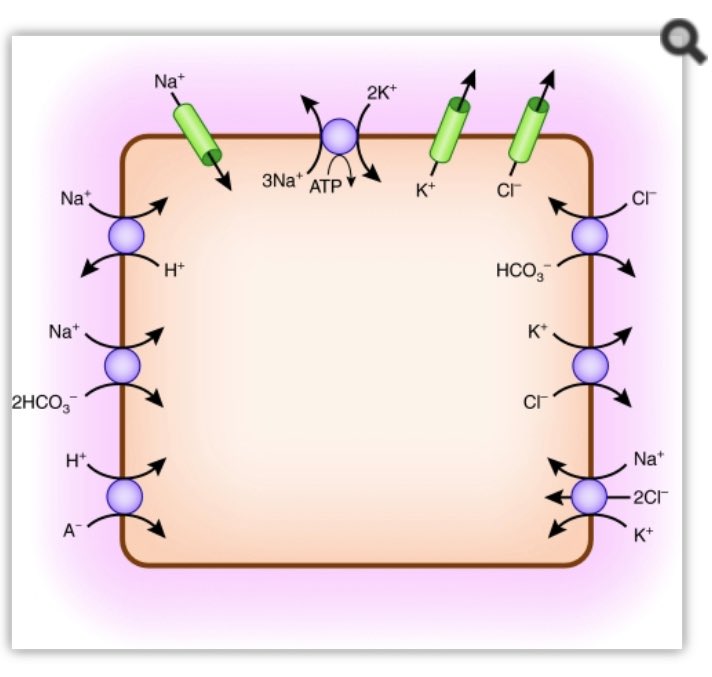
The distribution volume of K is far greater than the extracellular fluid volume. Any infusion with a physiologically appropriate K+ concentration will have minimal influence on serum K+ levels. Take a 50kg teenager for example. Assume our patient’s K+ =7mmol/L
What is the estimated total Extracellular fluid of our patient ( ECF is plasma plus Interstitial fluid plus transcellular fluid)
Now imagine we give him/her a litre of a theoretical solution containing 9 mmo/L of K+(remember this is a thought experiment don’t try irl)
Funny you should mention it, there are at least 3 papers I could find ,
The 1st study was published by O’Malley and colleagues
pubmed.ncbi.nlm.nih.gov/15845718/
shorturl.at/jF478
Don't factor in intraoperative K+ surge due to tissue damage/ apoptosis . Also the volumes given in the studies are very liberal, it is likely that smaller infused volume will cause less dramatic swings in K and pH.