“Large Scale Synthesis of Dysprosium and Neodymium Diiodides” (plus Thulium 💚).
pubs.acs.org/doi/abs/10.102…
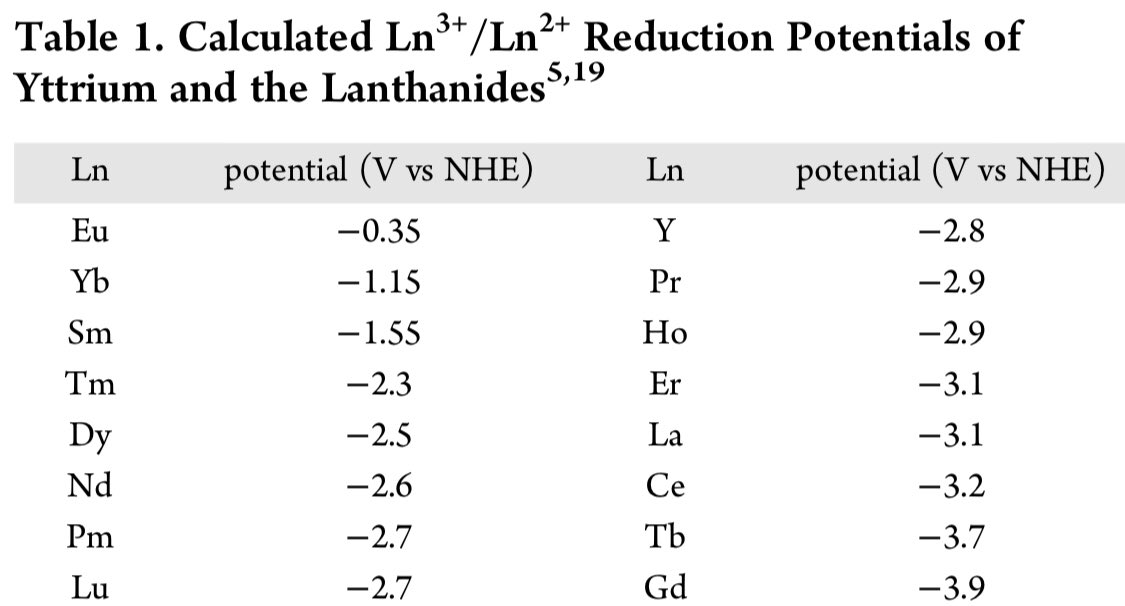
A thread #ChemTwitter #RealTimeChem (but from the past). (Table - pubs.acs.org/doi/10.1021/ja…)
Eu(II) has some pretty #Fluorescence!!

You can also use I-C2H4-I, or I2CH2.
You *can* make [TmI2(DME)3] from Tm(0) + TmI3 in boiling DME (onlinelibrary.wiley.com/doi/10.1002/an…) but it’s also temperature and light sensitive. It’s tough to get the timing right.
An alternative is to use a quartz reactor system where you conduct the synthesis in molten LnI2 that you carefully produce at first.
Here is where the Tm metal ignites when the I2 contacts it. We have a vast excess of Tm(0) at this point.
Making sure to “top up” the vacuum often.
Each I2 addition is pretty damn cool, heating the molten LnI2 up to a bright yellow glow.
Once everything is added, it’s cooled down under dynamic vacuum to remove excess I2.







