1/ After many questions, it’s time to clear the air on HOCl foggers, used in many dentist’s offices. @jljcolorado, @chemdelphine and I thought we’d go thru its chemistry. Bottom line from 3 Chem Profs: Use ventilation and filters to clean the air, skip the chemistry!
2/ HOCl is an oxidant that’s effective at killing pathogens, likely by denaturing proteins or damaging cell walls, bc it reacts w/ C=C bonds in many biomolecules. But HOCl can also react with YOUR biomolecules (lung, skin), material surfaces, and the molecules that make up air.
3/ HOCl is good for disinfecting surfaces and drinking water, but fogging rooms that people may re-enter after a short wait raises big chemical exposure questions. Our stomach copes with chemicals different from our lungs! “The dose makes the poison,” but so can the route!
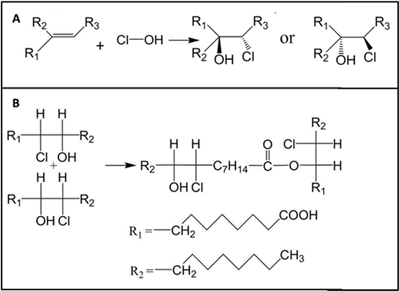
4/ HOCl reacts with unsaturated (C=C containing) organic compounds to produce chlorohydrins and reactive oxygen species, including free radicals. Some chlorohydrins are highly toxic. Chlorohydrins can react with each other to form dimers (as an ester). pubs.acs.org/doi/10.1021/ac…
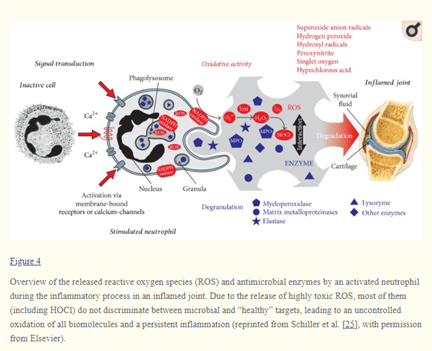
5/ HOCl is produced by our bodies in inflammatory responses, but introducing this potent oxidant where it’s not meant to be can have important negative effects. Reaction with some lipids can contribute to inflammatory disease or oxidative stress.
ncbi.nlm.nih.gov/pmc/articles/P…
ncbi.nlm.nih.gov/pmc/articles/P…
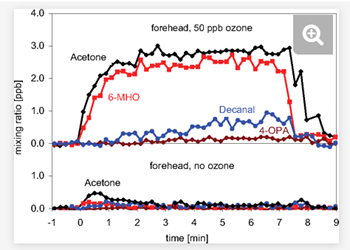
6/ The reaction of HOCl gas with a film of squalene (from skin oil) is fast enough to be impactful even in a well-ventilated room. The reaction rate is similar to the more heavily studied reaction with ozone (O3). For info on O3, see this key paper pnas.org/content/107/15…
7/ Ozone is well-known to be a pollutant and respiratory hazard (). HOCl reacts with similar compounds, at a similar rate, with similarly concerning products. Why is HOCl widely considered to be harmless? epa.gov/ground-level-o…
8/ HOCl is found at very low concentrations outdoors - and most air quality studies focus on outdoor air. But what about indoor air, where HOCl could be introduced strongly and reach high concentrations? We need more studies to be asking these types of questions.
9/ Mopping with bleach releases HOCl gas that reacts with indoor gases, light, surfaces and airborne particles to produce an array of chemicals. We are not only worried about exposure to HOCl itself, but also products of its chemical reactions. pubs.acs.org/doi/full/10.10…
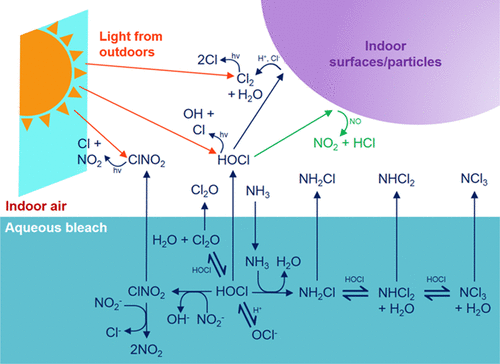
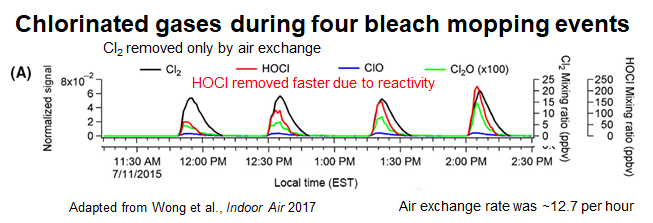
10/ HOCl reactions are plenty fast enough to outpace air exchange, so they’re certainly important for indoor air. Below, in 4 bleach moppings, Cl2 gas is being flushed out by air exchange in this case, HOCl gas goes away faster -- it’s chemically reacting! onlinelibrary.wiley.com/doi/full/10.11…
11/ Lots of people know about the chlorine in bleach, sodium hypochlorite (NaOCl). Hypochlorite salts dissolve well in water (making Na+ and OCl- ions) and this salt does not evaporate, but if the acid form (HOCl) is present, it *can* evaporate. This is key.
12/ In any hypochlorite solution, the amount of OCl- and HOCl is in ‘chemical equilibrium’ - both exist in a set ratio. The value of the ratio depends on the pH of the solution (how many H+ ions are present). Bleach is a basic solution (pH~11), so OCl- dominates. 
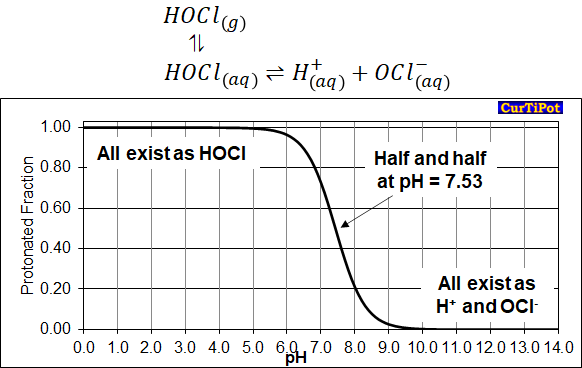
13/ The chemistry of HOCl foggers is altered compared to regular bleach so that HOCl > OCl- in solution (low pH). The amount of HOCl that can evaporate from fogging is much much higher than from bleach. And indoor chemistry with bleach is pretty noxious to start with!
14/ HOCl can react with molecules present in the indoor environment to produce toxic gaseous products. For example, Cl2 gas is formed from reactions with chloride, HOCl reacts with ammonia and amines to produce chloramines - both are known to be harmful. cdc.gov/healthywater/s…
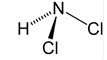
15/ From the #HOMEChem project, a study by @jimmymattila: HOCl reacts with VOCs (eg, isoprene and limonene) to produce oxygenated VOCs, chloroacids, and isocyanates. HOCl gas reacts with chemicals deposited on surfaces in the room.
pubs.acs.org/doi/full/10.10…
pubs.acs.org/doi/full/10.10…

16/ HOCl chem also spurs ultrafine airborne particles to form and adds to the growth of particles in indoor air. We know from outdoor studies (incl. Harvard 6 cities study, below) that airborne particles induce respiratory and cardiovascular issues. nejm.org/doi/full/10.10…
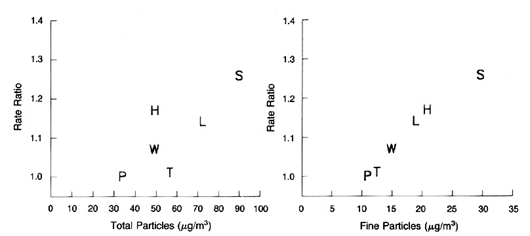
17/ Studies at @envchemuoft showed huge production of pollutant particles in air when HOCl reacts with limonene, the citrus scent added to cleaning products and perfumes (abundant indoors overall). pubs.acs.org/doi/10.1021/ac…
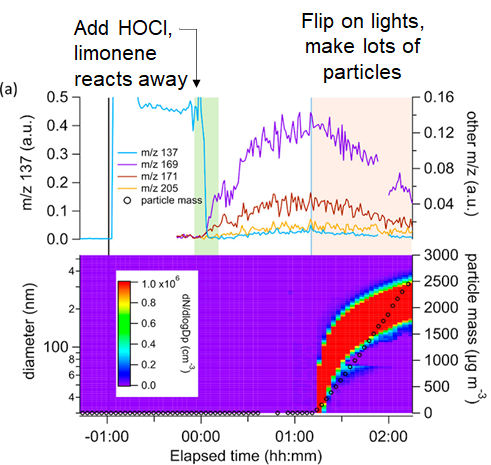
18/ Seen in #17, gas-phase oxidation by HOCl can happen in the dark, but when fluorescent lights are turned on (or sunlight thru window) the chemistry changes -- more airborne particles were created (by mass) than in Los Angeles on a smoggy day w/ similar HONO level to foggers. 

19/ Fogging might work between uses of a room, but still need to wait for clean air to replace the HOCl-laden air. In one air-change with outdoor air, the pollutant is reduced to 36% of the original amount, still too high - need to wait 3 air exchanges to get down to 5%
20/ The air change rate in dentist’s offices probably varies a lot. One study () measured ~5 ACH in a real office. When using HOCl foggers, wait time between patients should then be 3/5 h = 36 min. sciencedirect.com/science/articl…
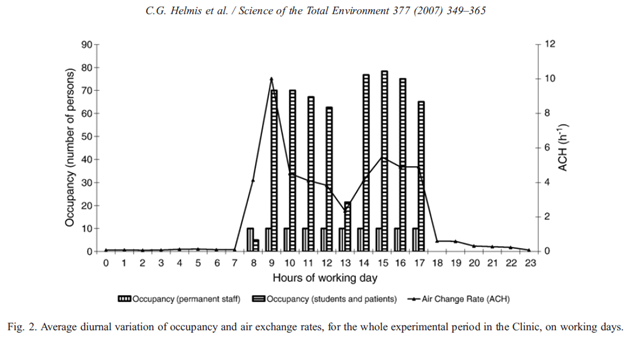
21/ quotes 8-12 as typical values for dental offices, so wait times in that case would be 15-23 min. Are these wait times being used, and do dental offices know their air change rate?engineeringtoolbox.com/air-change-rat…
22/ See this post for a way to measure your own air change rate: )medium.com/@jjose_19945/h…
23/It takes 3 air changes to clear 95% of the chemicals from the air. But if the chemicals are cleared, so are the virus particles! There is no advantage to fogging compared to just waiting between uses, other than surface disinfection - there are safer ways to disinfect surfaces
24/ Using HOCl foggers between dental patients ignores the idea there are easier/safer ways to clean the air. Bring filtered air from outdoors through HVAC systems. Run these systems all the time to minimize exposure to hazardous chemicals & airborne virus-containing particles
25/ We recommend instead to use $$ on HEPA air cleaners or makeshift fan-filter cleaners: proven to work, easy to use, no side effect. And in a dental office, they work where needed most, **when the patient is in the chair**, not only between patients.
26/ See here for additional discussion of other air cleaners:
https://x.com/jljcolorado/status/1291758303089852417
27/ Going forward, we recommend either “localized HEPA cleaning” or “localized air extraction” to be installed in dental offices, where many “aerosol generating procedures” take place. Take the air near the patient’s mouth, and filter it or exhaust it.
28/ We have at least months left with this pandemic, and there may be others in the future, and also ongoing respiratory diseases like the flu -- so let’s get safe, effective air quality technology installed for the long run.
29/ We use localized extraction above our kitchen range, why not have an extractor (or ‘snorkel’) running during dental procedures? Here’s a commercial example that is seeing increasing use in chem labs 

30/ But there are less expensive, do-it-yourself ways -- Here’s an example of a hospital-scale version of a localized extractor setup made from common materials & limited cost:
31/31 Bottom line: enhance ventilation, use portable HEPA or makeshift filters, and avoid the HOCl foggers
Comments? Things we forgot to say? Did we miss or misrepresent something? If so, please send us any peer-reviewed references that support other points of view.
Comments? Things we forgot to say? Did we miss or misrepresent something? If so, please send us any peer-reviewed references that support other points of view.
PS - We were able to put this together because of some great research on the subject, including the likes of @JimmyMattila, @JFaustDoesChem, Chen Wang at @EnviroChemUofT, and the #HOMEChem @IndoorChem team. But we still have lots of work to do!
An accuracy update, HOCl is more reactive with -SH groups (in amino acids/proteins) and amines than C=C bonds.
See an update and more developed perspective on spray disinfectants in my #IndoorChem blog post, linked in the tweet below. #IAQ
https://twitter.com/EarthMechanic/status/1341086583517278215
• • •
Missing some Tweet in this thread? You can try to
force a refresh





