1/ Here are the clinical outcomes of the outpatient RCTs avaliable so far. Relative reductions (of HCQ vs control groups) in Y axis, p-values in X axis. Includes both prophylaxis and early treatment RCTs. At this point there's enough data for metanalyses. 

2/ Here's a spreadsheet with notes and the plot itself. Pay special attention to the "Notes" column in the "summary_table" sheet:
hcq-outpatient-rcts.s3.amazonaws.com/outpatient_hcq…
hcq-outpatient-rcts.s3.amazonaws.com/outpatient_hcq…
3/ As more outcome data comes in, one can aggregate and analyze the data jointly. This is what a "metanalysis" is. In the case of outpatient HCQ RCTs, since the effects on symptoms are consistently positive, lo and behold!, the p-value shrinks and eventually crosses p<0.05
4/ Two metanalyses of the clinical outcomes are in.
First, one using all clinical outcomes by Harvey Risch from Yale and coauthors:
medrxiv.org/content/10.110…
First, one using all clinical outcomes by Harvey Risch from Yale and coauthors:
medrxiv.org/content/10.110…
5/ Second, the metanalysis by Xavier Garcia-Albeniz of Harvard and coauthors, using the clinical outcomes only of the prophylaxis RCTs:
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…
6/ The two metanalyses reach similar conclusions, as indeed they must, since they're both essentially calculations on the outcomes shown in the plot, which are quite robust: about a 20% relative reduction in symptoms when HCQ is used either early or prophylactically
7/ Both rely on random-effects models, a standard statistical technique tailored to jointly analysing different outcomes:
en.wikipedia.org/wiki/Random_ef…
en.wikipedia.org/wiki/Random_ef…
8/ A lot more to say here. The viral load results, differently from the clinical effects, are all over the map: sometimes positive, sometimes negative, generally hovering around zero. Therefore consistent with HCQ having *no relevant antiviral effect in vivo*.
9/ So HCQ's in vitro antiviral effect seems looks like an accidental red herring. The clinical effect is likely immonumodulatory.
In vivo antiviral effects: ambiguous effect on PCR in Rajasingham, none on viral load in Mitjà CID, very marginal effect on PCR+ in Abella
In vivo antiviral effects: ambiguous effect on PCR in Rajasingham, none on viral load in Mitjà CID, very marginal effect on PCR+ in Abella
10/ The outcome from Abella not shown in plot because it is based on viral load, not on symptoms: it measures conversion from PCR- to PCR+. That is, it is not a clinical outcome. In the plot its outcome would hover just above X axis in far right.
11/ Abella is extremely underpowered for a pre-exposure prophylaxis RCT, with only 64 patients in treatment group and 61 in placebo group, but nevertheless has useful data on the (negligible) Q_t prolongation effect of HCQ, see picture. 

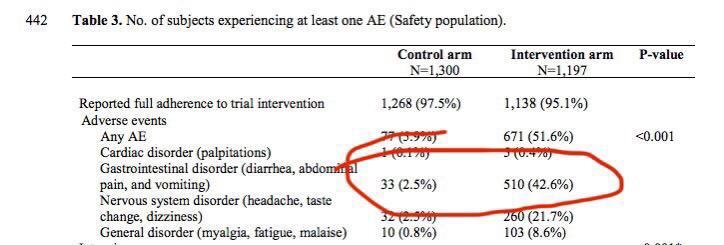
12/ So yeah, barring specific contraindications outpatient HCQ is very safe, as it has always been (dispensed to millions of rheumatic patients, largely elderly, over decades with no need for eletrocardiograms etc etc). Diarreah and nausea are the only common side effects.
13/ There's been all sorts of madness around this treatment. Some examples:
13.i. In the media a few of the authors are publicly fighting the data from their own studies This is the p < 0.05 bright-line fallacy raised to the level of lunacy.
13.i. In the media a few of the authors are publicly fighting the data from their own studies This is the p < 0.05 bright-line fallacy raised to the level of lunacy.
13/ More HCQ madness:
13.ii. The Mitjà preprint included a large share of PCR positive patients *at the baseline*. Once those are removed, the effect is the 29.9% relative reduction shown in plot. (Prior to removal, there's a relative reduction of 11%)
13.ii. The Mitjà preprint included a large share of PCR positive patients *at the baseline*. Once those are removed, the effect is the 29.9% relative reduction shown in plot. (Prior to removal, there's a relative reduction of 11%)
13/ Yet more HCQ madness:
13.iii. Also in Mitjà post-exposure prophylaxis preprint, the "secondary outcome" includes diarrhea as a *sufficient* condition for diagnosis of "covid positive". And HCQ induced diarrhea in 42% of treatment group. So the HCQ group had "more covid"...
13.iii. Also in Mitjà post-exposure prophylaxis preprint, the "secondary outcome" includes diarrhea as a *sufficient* condition for diagnosis of "covid positive". And HCQ induced diarrhea in 42% of treatment group. So the HCQ group had "more covid"...
14/ What about HCQ for hospitalized patients? Ineffective, based on RCT data. HCQ decreases symptoms if taken while symptoms still benign. If they worsen significantly, better rush to hospital and there be treated with steroids / remdevisir / blood thinners etc as called for.
15/ Note that hospitalized cohorts suffer from "adverse selection". They are precisely those people whose symptoms became severe enough to go to hospital (and be admitted as inpatients). These are the cohorts that hospitalized RCTs analyze.
16/ Overall takeaways from outpatient HCQ data:
16.i. taken early, reduces symptoms / hospitalization / death by about 20%
16.ii. barring specific contraindications, very safe, as it has always been
16.i. taken early, reduces symptoms / hospitalization / death by about 20%
16.ii. barring specific contraindications, very safe, as it has always been
16/ More overall takeaways from outpatient HCQ data:
16.iii. In the Skipper AIM and Mitjà CID early outpatient RCTs treatment began (including shipping delay) on average 4-5 days after symptom onset, so effect likely somewhat > 20% reduction if taken on say day 2 or 3 instead
16.iii. In the Skipper AIM and Mitjà CID early outpatient RCTs treatment began (including shipping delay) on average 4-5 days after symptom onset, so effect likely somewhat > 20% reduction if taken on say day 2 or 3 instead
16/ Yet more overall takeaways from outpatient HCQ data:
16.iv. In "real world", outpatient treatment has generally involved HCQ combined with other drugs (e.g., azithromycin or invermectin). No RCT data on those combinations yet, but observational data suggest stronger effects.
16.iv. In "real world", outpatient treatment has generally involved HCQ combined with other drugs (e.g., azithromycin or invermectin). No RCT data on those combinations yet, but observational data suggest stronger effects.
@threadreaderapp unroll
@_MiguelHernan, see points 8 through 11. They're very relevant to prophylaxis studies.
@BallouxFrancois, see thread: you're right, effect is on symptom severity and progression, not contagion per se. And must be taken early.
@jwato_watson, see thread. By now picture is clear from RCT data. It's not just about prophylaxis, in fact the effect is not prophylaxis at all, strictly speaking.
@jpkiekens, early HCQ doesn't seem to act as an antiviral in vivo, but does reduce symptoms, see thread
(My apologies to all for the serial tagging. This is an important subject, and several nuances from the outpatient RCT data have not been picked up by many medical researchers)
• • •
Missing some Tweet in this thread? You can try to
force a refresh