How and why I treat high risk smoldering multiple myeloma (SMM). Thread.
1/ Based on the progression risk curve over time, and genomic analysis, SMM is now considered a heterogenous clinical entity in which 50% of patients have premalignancy and 50% asymptomatic malignancy.

1/ Based on the progression risk curve over time, and genomic analysis, SMM is now considered a heterogenous clinical entity in which 50% of patients have premalignancy and 50% asymptomatic malignancy.
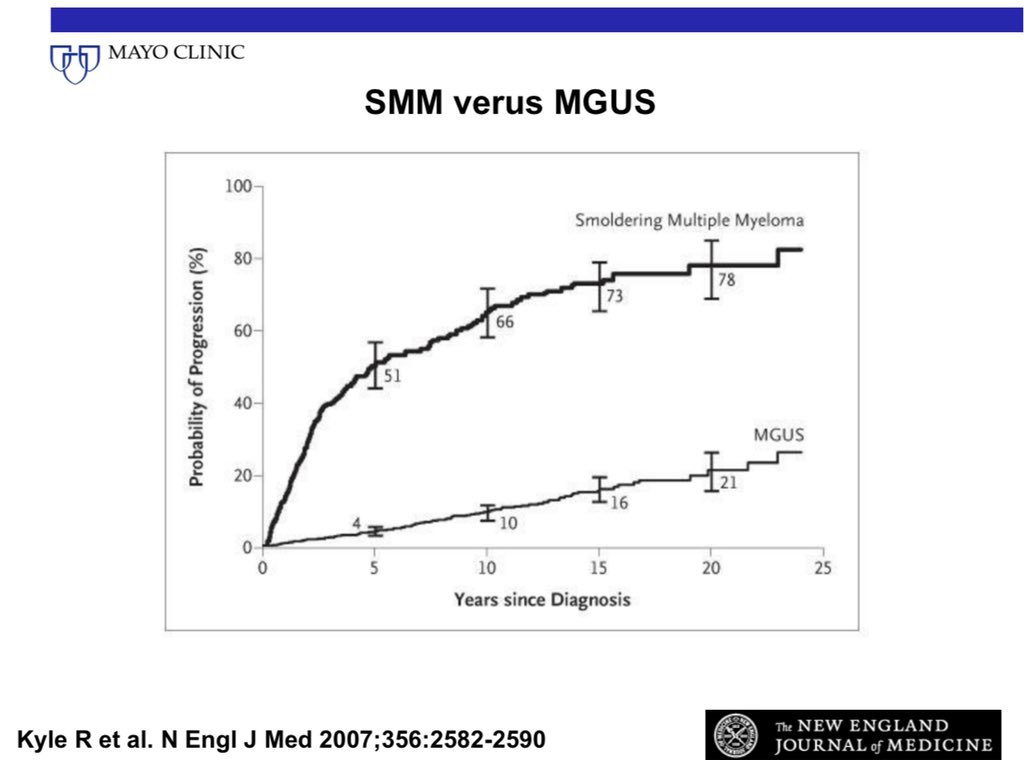

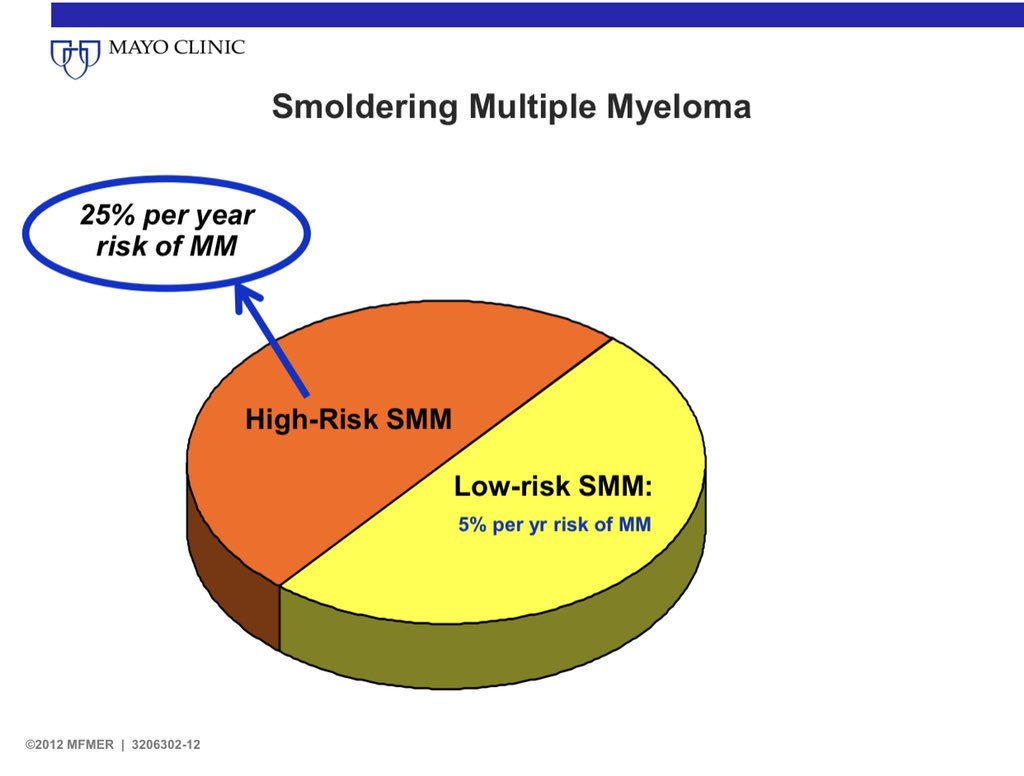
2/ These two groups can be considered as high risk SMM (asymptomatic malignancy) and intermediate/low risk SMM (premalignancy).
We are specifically concerned about high risk SMM, defined as 50% risk of progression to myeloma over 2 years.
We are specifically concerned about high risk SMM, defined as 50% risk of progression to myeloma over 2 years.
3/ Patients with high risk SMM can be identified by the Mayo 2018 criteria: Also called the 20-2-20 criteria.
BMPC >20%
M spike >2
FLC Ratio >20
Any 2 of the 3 risk factors is considered high risk SMM. @MayoMyeloma @MayoCancerCare @myelomaMD
BMPC >20%
M spike >2
FLC Ratio >20
Any 2 of the 3 risk factors is considered high risk SMM. @MayoMyeloma @MayoCancerCare @myelomaMD
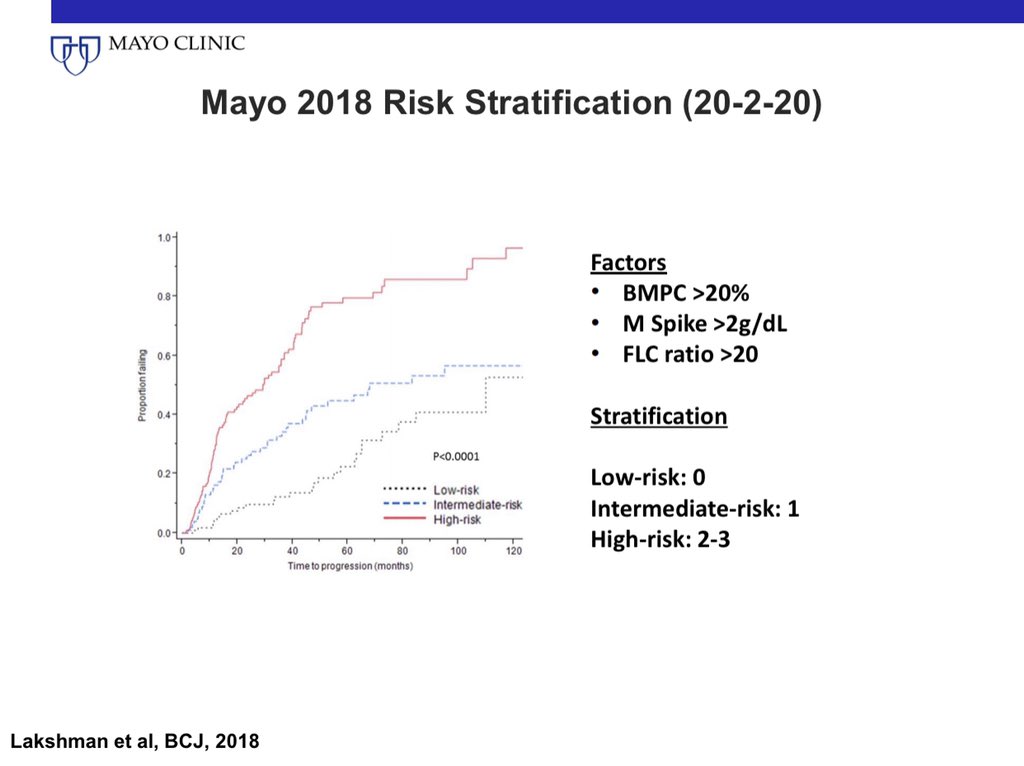
4/ The Mayo 2018 criteria have been validated by the IMWG.
One third of all patients with SMM have high risk SMM.
One third of all patients with SMM have high risk SMM.

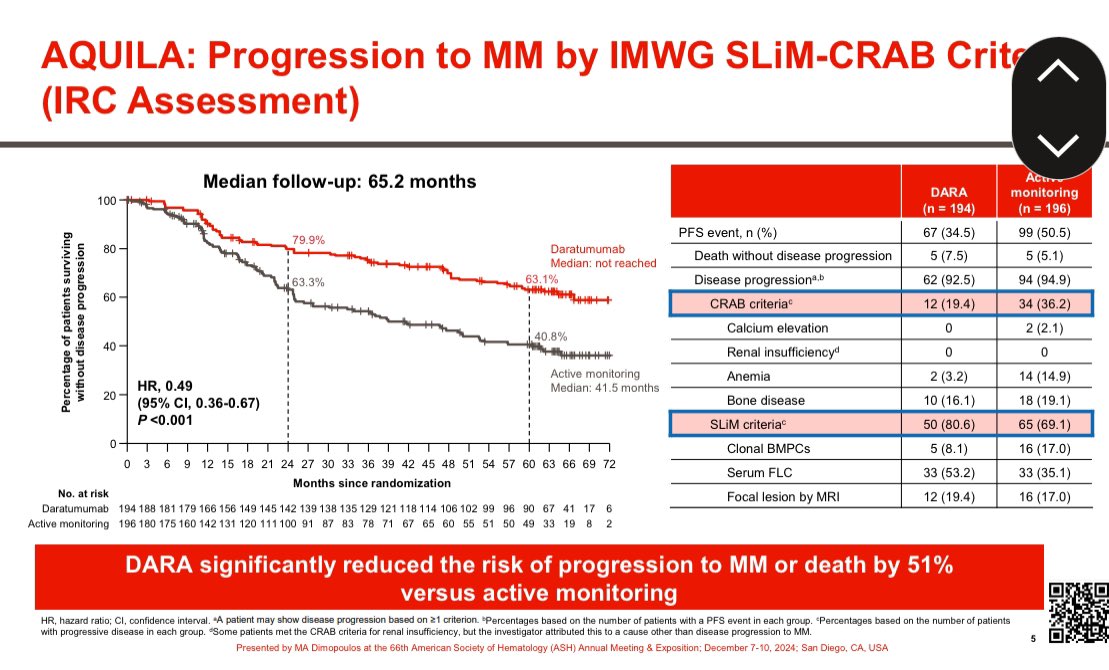
5/ The Spanish RCT of Len/dex vs Observation found benefit with early therapy in high risk SMM in terms of time to end organ damage and overall survival. Risk reduction for progression was 90% in the Mayo 20-2-20 high risk group. @mvmateos 



6/ A 90% reduction in time to end organ damage was also seen in the @eaonc RCT of Len vs Observation. Only 6 patients have died in this trial: 2 in the Len arm and 4 in the observation arm. It will take many years to comment on OS. @SagarLonialMD 

7/ The question of whether high risk SMM is best treated with mild therapy with Len or Len/dex or with a myeloma like triplet regimen is being studied in the ongoing @eaonc trial. @nsc_natalie @mtmdphd @NorthTxMSG 
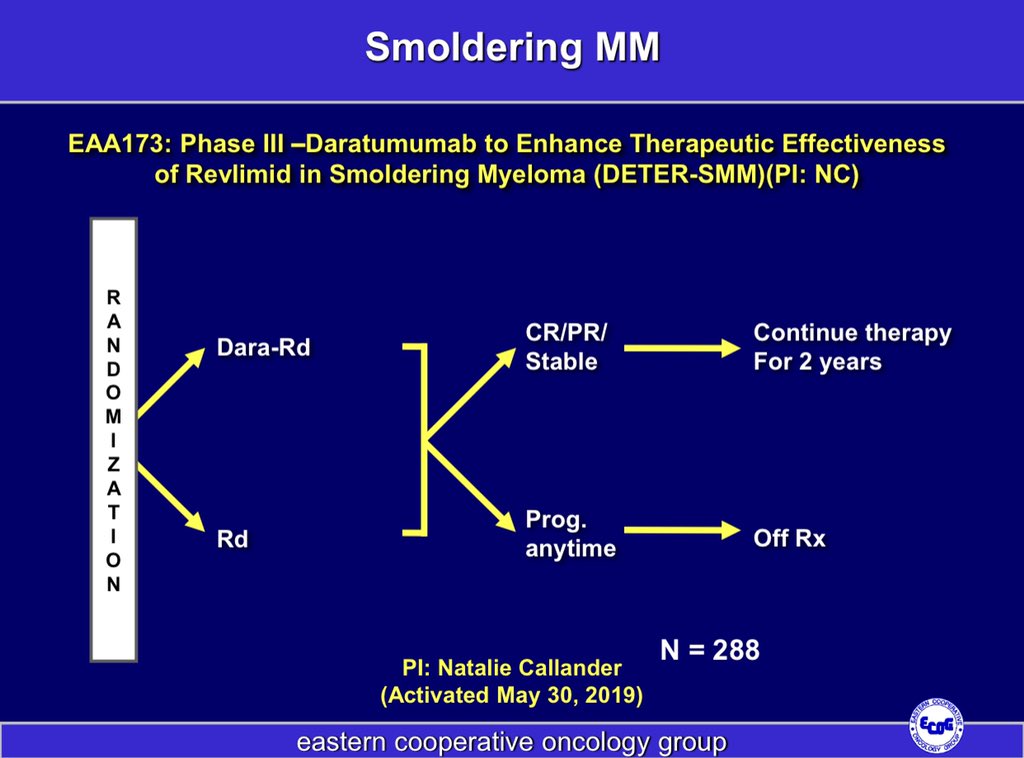
8/ We have 3 types of trials in SMM.
1) The "necessary" trials for regulatory purposes to show something is better than nothing.
2) The strategic trials to improve outcome
3) The trials aiming to cure.
We are doing these in parallel.
1) The "necessary" trials for regulatory purposes to show something is better than nothing.
2) The strategic trials to improve outcome
3) The trials aiming to cure.
We are doing these in parallel.

9/ Based on what we know so far, I recommend either clinical trials or early therapy with Len or Len/dex for 2 years in newly diagnosed high risk SMM provided such treatment is feasible in a given country.
There are no more RCTs of treatment vs Observation ongoing.
There are no more RCTs of treatment vs Observation ongoing.

10/ It is extremely hard to do an RCT in this patient population. I have led 4 of these with my colleagues. I don't make recommendations like this without considerable thought on the pros and cons.
11/ If more details are available at diagnosis, the IMWG scoring system provides a better estimate of risk that can be used to risk stratify patients, for counseling, and management. @mvmateos @myelomaMD 

12/ If patients with SMM are being observed without treatment, a rise in M protein accompanied by a fall in hemoglobin by >0.5 gms is associated with a high risk of progression. If BMPC is >20%, risk in these patients is 90% at 2 years. 
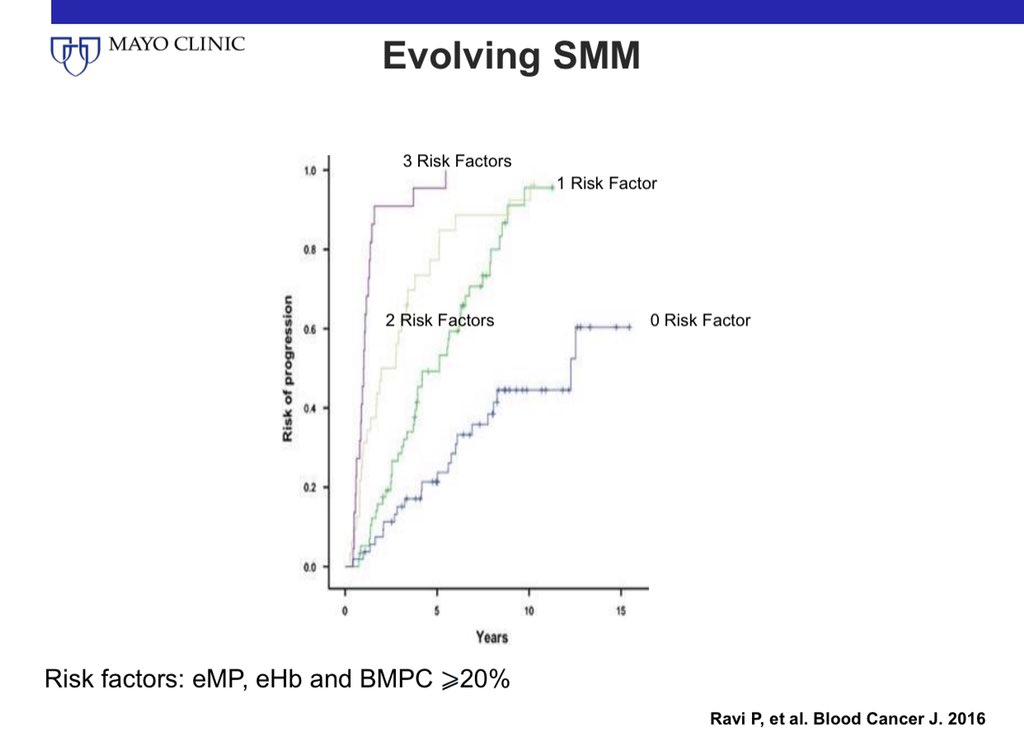
Some have said they prefer clinical trials. I do too. My algorithm is if trials are not available.
Note all clinical trials currently accruing/planned do not have an observation only arm. There will be no further data on OS between treatment vs observation for several years.
Note all clinical trials currently accruing/planned do not have an observation only arm. There will be no further data on OS between treatment vs observation for several years.
• • •
Missing some Tweet in this thread? You can try to
force a refresh