In a recent thread I posited that mechanisms of antibody production, viral resistance, and somatic hypermutation can explain why pre-Delta vaccines block most Delta infections but not peak virus levels once infected, yet still limit late disease.
Here I present the explanation…
Here I present the explanation…
https://twitter.com/michaelzlin/status/1431418682144481285
Most neutralization of SARSCoV2 infection is performed by antibodies against the spike (S) protein, and the vaccines used in the US include only S and no other parts of SARSCoV2, so we'll concentrate on S as the antigen, i.e. the protein targeted by antibodies or T cell receptors 

Your body has millions to billions of resting/naive B cells, each with an unique surface-bound antibody or immunoglobulin (Ig), just waiting to find their match to foreign particles. You also have T cells with unique T cell receptors (TCRs)
ncbi.nlm.nih.gov/pmc/articles/P…
ncbi.nlm.nih.gov/pmc/articles/P…
Upon vax or SARSCoV2 infection, those lucky naive B cells with surface Ig that bind S become partly activated. They also internalize and dice up S into small bits (red dot belows) to load onto MHC2 molecules and present to TCRs on T cells. (Figure from researchgate.net/publication/32…) 

If a T cell recognizes a small bit of S presented by the B cell (on MHC2 molecules) then it fully activates the B cell to replicate. The progeny then mature into Ig-secreting plasma cells or memory B cells that mutate the Ig more (discussed later)
(Fig: researchgate.net/publication/30…)
(Fig: researchgate.net/publication/30…)

Now to prevent respiratory viruses from establishing an infection, antibodies have to act on viruses as they land on the mucous of your mouth, nose, and throat. These antibodies are supposed to be IgA, which exists in dimeric form. I've made a picture of that below. 
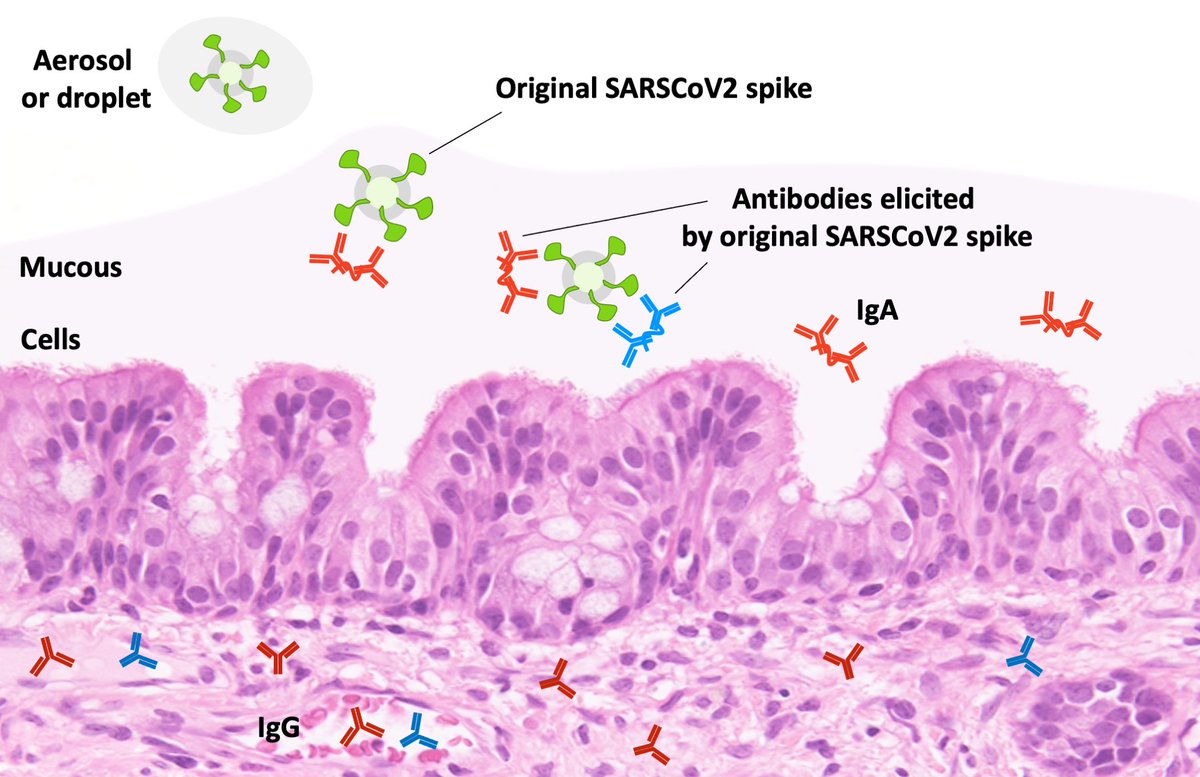
But concentrations of Ig from those with current vaccines (with original S) need to be 5x higher to neutralize Delta vs original SARSCoV2. Is it because binding to all antibodies is reduced to 20%, or 80% of neutralizing antibodies don’t bind at all while 20% are unaffected?
Given that multiple antibodies bind to multiple parts of spike, it's not likely for Delta mutations to reduce each binding reaction to 20% on average. More likely 80% on average of the neutralizing antibodies are rendered completely inactive. 
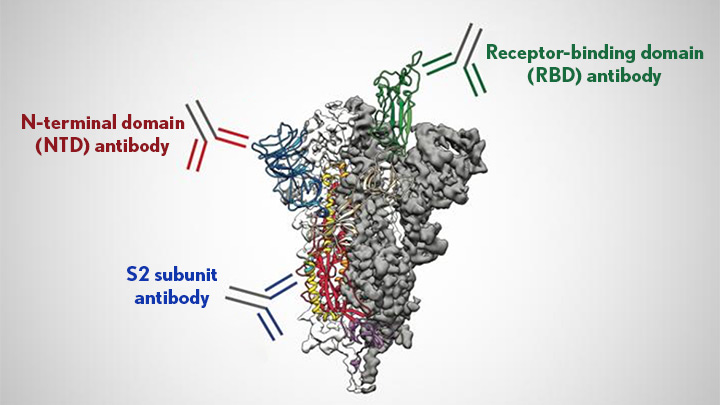
A study using monoclonal antibodies showed that Delta mutations only abolish antibody binding at one of several possible neutralizing sites. But mutations in Delta must be hitting the sites that are recognized by 80% of the average antibody mix. nature.com/articles/s4158… 
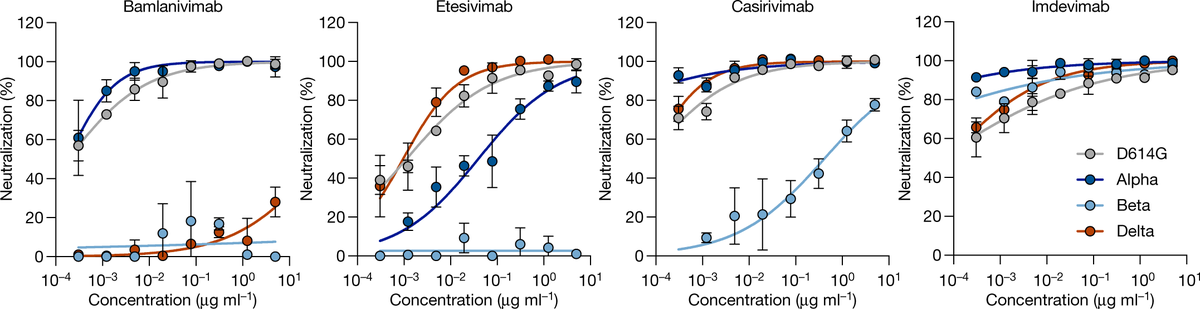
Note these numbers are averages, each individual's response won't be exactly the average.
Anyway, the ~5x fewer antibodies to Delta spike vs original spike reduces protection vs infection from 95% to ~85% per measurements of vaccine effectiveness from the UK.
Anyway, the ~5x fewer antibodies to Delta spike vs original spike reduces protection vs infection from 95% to ~85% per measurements of vaccine effectiveness from the UK.
It makes sense that the drop seems modest: 80% of your antibodies now don’t do much, but the remaining 20% are enough to prevent infections from most encounters. With a short encounter, antibody molecules still match virus particles locally when the viruses land. 

But dose matters: if you share an enclosed space with a highly contagious person for a long time, you might breathe in so many particles that some get past the antibody picket line.
In the 15% of the time viruses pass through mucosal IG, though, then things get temporarily out of control. Hundreds thousands of new viruses are produced from every infected cell. These new virus particles now outnumber antibodies locally, allowing many to infect more cells.
Existing plasma cells can be activated to produce more Ig, and T cells and macrophages/monocytes come to eat up infected cells (T cells recognizing them via TCR-MHC1 interactions and macrophages/monocytes decorated with Ig on their Ig receptors), pic from nature.com/articles/s4157… 

For original SARSCoV2, Ig from existing plasma cells eventually suppresses virus to lower levels than unvaccinated (per studies in thread in the first post). But apparently Delta's faster propagation and Ig evasion allows it to achieve levels similar in vaxxed and unvaxxed.
Why then do vaccinated people suppress virus after 1 week faster? Some naive B cells matching Delta spike but not original spike will be activated, but that will occur similarly in vaxxed and unvaxxed. Two other thngs that are happening are different between vaxxed and unvaxxed.
First, the vaccinated/prev infected do have the existing long-lived plasma cells still making the good fraction of antibodies. So these can ramp up production after being restimulated. pubmed.ncbi.nlm.nih.gov/30747835/
Second, vaccinated and previously infected have memory B cells that can now differentiate into plasma cells, and can make antibodies that better match Delta via somatic hypermutation (SHM), where Ig genes are randomly mutated.
Where did these memory B cells come from? On initial vax or infection, some of the stimulated B cells became memory B cells rather than plasma cells. It's thought these may be those B cells with lower-affinity Ig. They continue to express surface Ig while mutating them (my pic) 
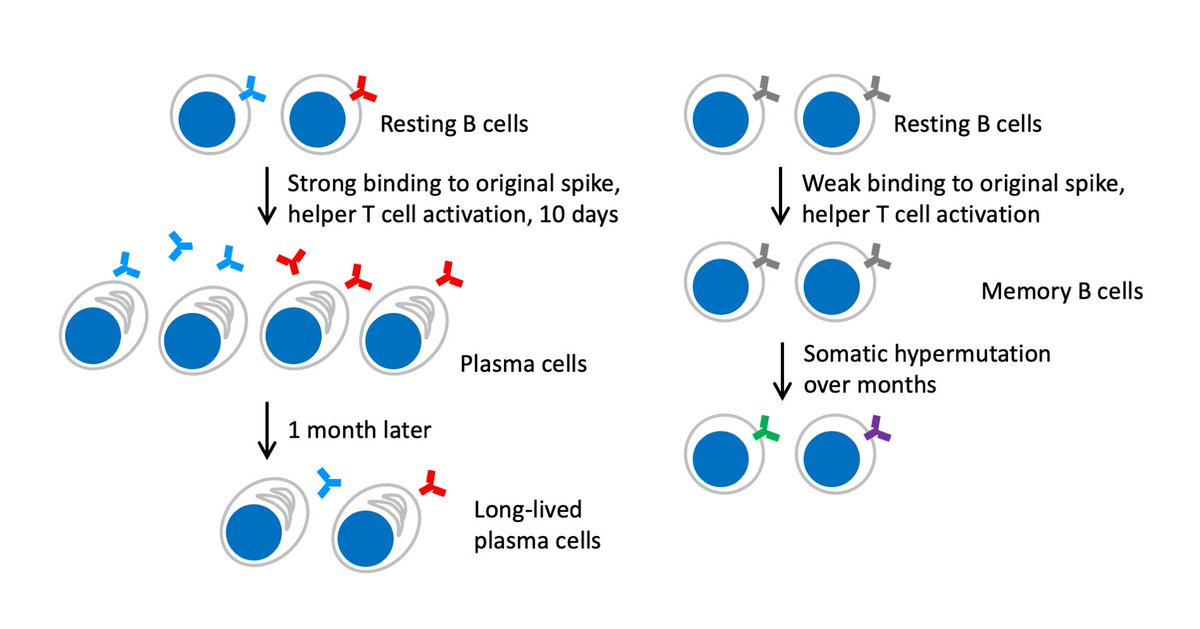
The process of somatic hypermutation of those Ig genes takes place for months after the initial immunization event.
nature.com/articles/s4158…
nature.com/articles/s4158…

Then upon Delta infection, existing plasma cells make more Ig, and memory B cells that express surface Ig matching Delta spike proliferate and differentiation into new plasma cells. These two events explain why vaccinees still respond better than unvaxxed, but with a 1wk delay. 

And how about T cells? Cytotoxic T lymphocytes (CTLs) that recognize and kill virus-infected cells are an important part of the response. They are likely, in concert with viral neutralization by Ig, to prevent viral spread to organs and thereby severe disease and death.
But CTLs recognize many segments in the virus (in natural virus or inactivated virus vaccines) or in the spike protein (in S-only vax). So far no viral immunoevasion is attributed to a change in CTL recognition, so CTLs explain commonalities rather than differences in response.
In addition, while you need antibodies to inactivate the millions of virus particles in an infection, CTLs act on cells and not directly on viruses. Thus IMO T cells alone could not end a viral infection; antibodies must be ramped up to limit circulating virus.
Still T cells in the vaccinated certainly contribute to the clearance of infections as well. But again any effect is only noticeable after the first week if you are infected with Delta.
Given that Delta is more contagious and leads to more severe disease (as found in Scotland in June and recently confirmed in England below), the vaccines that use Delta spike rather than original spike would be of huge benefit. nytimes.com/2021/08/27/hea…
In any case, the ability of humoral (antibody-mediated) immunity to re-adapt, via memory B cells and somatic hypermutation, likely work together with T cells to clear Delta more quickly in vaxxed vs unvaxxed after the first week.
• • •
Missing some Tweet in this thread? You can try to
force a refresh










