16 November: The right side of the curve
For Lung Cancer Awareness Month #LCAM I’m going to summarize 30 important lung cancer trials over 30 days. These posts are directed at non-medical professionals, with descriptions of the results and of what makes a good trial. #lcsm 1/14
For Lung Cancer Awareness Month #LCAM I’m going to summarize 30 important lung cancer trials over 30 days. These posts are directed at non-medical professionals, with descriptions of the results and of what makes a good trial. #lcsm 1/14

In our previous discussion of immunotherapy (13 Nov) we talked about the expression of PD-L1 on tumour cells. At the time of this study (2014) there was some evidence that tumours with more cells expressing PD-L1 were more likely to benefit from immunotherapy. 2/14 

This trial enrolled people with metastatic lung cancer (non-EGFR, non-ALK) where >50% of tumour cells expressed PD-L1. About one third of NSCLC meet this criterion. They were randomized to either standard chemotherapy, or to pembrolizumab immunotherapy for up to two years. 3/14
Primary outcome was time to cancer worsening (PFS). There was a very complex interim analysis scheme that ultimately stopped the trial due to evidence of a survival advantage with pembrolizumab. Here are the initial KM curves showing PFS. 4/14 
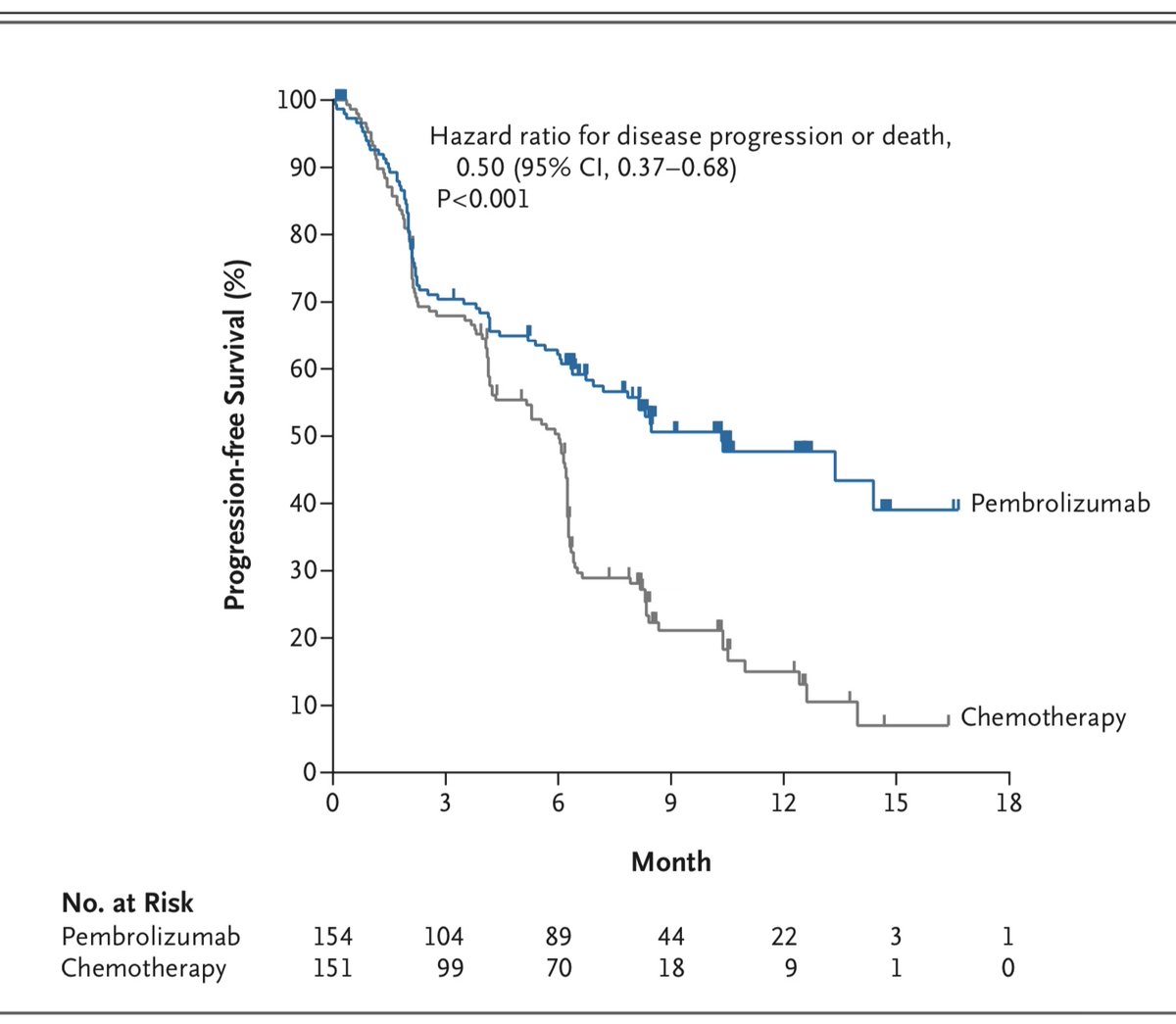
The overall survival results have been updated in a subsequent paper. I think it's striking that the median PFS is around 9 months, yet the median OS is 30 months! 5/14 

Considering the previous trials we’ve looked at, we know that chemo-immuno is better than chemo in first line regardless of PD-L1 expression.
We know from today’s trial that immuno is better than chemo if PD-L1>50%. Of course it’s a different story if EGFR or ALK positive. 6/14
We know from today’s trial that immuno is better than chemo if PD-L1>50%. Of course it’s a different story if EGFR or ALK positive. 6/14
It remains unknown if there are subgroups of PD-L1>50% where chemo-immuno is better than immuno alone.
It is also unknown if there are groups of PD-L1<50% where immuno alone might be better than chemo/immuno. 7/14
It is also unknown if there are groups of PD-L1<50% where immuno alone might be better than chemo/immuno. 7/14
Let’s take a look at the KM curve here. We previously looked at these in detail on 5 November. There are aspects of these curves we have not considered. Take a look at the numbers along the bottom. 8/14 

“No. at Risk” is the number of participants followed to that time point. There are only 5 people in each arm who’ve been followed to 30 months. Look at all the censored people between 21-30 months: they were enrolled in the last 9 months of the trial and are still in follow-up. 

Look at the big drop in the pembro arm right around 30 months. This large steps happens because there are only 6 people followed to that time. When one of them dies, the curve drops down one sixth of the distance to zero. (in this case from 52% to about 43%)
10/14
10/14

All of this is pertinent, because people tend to focus on the part of the KM curve furthest to the right. This gives us information about long-term survivors, and if the curve is flat for a long period of time, we speculate about people being “cured”. 11/14
But this is the least certain part of the curve. Its shape is determined by the smallest number of people, and events in a few drastically alter how this part of the curve looks. 12/14
We can’t help but look with interest at the right-most part of the curve. We can be cautious, though, about over-reading it. 13/14
Tomorrow we’ll be back to adjuvant chemo for NSCLC, and we will wrestle again with the question of which analyses you can take to the bank, and which ones are not so sure... 14/14 

• • •
Missing some Tweet in this thread? You can try to
force a refresh