18 November: Pemetrexed & placebo
For Lung Cancer Awareness Month #LCAM I’m going to summarize 30 important lung cancer trials over 30 days. These posts are directed at non-medical professionals, with descriptions of the results and of what makes a good trial. #lcsm 1/13
For Lung Cancer Awareness Month #LCAM I’m going to summarize 30 important lung cancer trials over 30 days. These posts are directed at non-medical professionals, with descriptions of the results and of what makes a good trial. #lcsm 1/13

Today’s trial takes us back to 2005, when standard first-line platinum doublet was given for 4-6 cycles, followed by a treatment break. People would be followed, usually for a few months, and when their cancer worsened they would get second-line chemo, often pemetrexed. 2/13
This trial asked the question of whether people would live longer if the pemetrexed started immediately after first-line chemo (maintenance treatment), rather than waiting and using it in second line. At the time of this study, pemetrexed was not used in first-line. 3/13
People were eligible for the trial if they had four cycles of platinum doublet chemo and their cancer did not get worse. 663 people were randomized 2:1 to either pemetrexed or placebo. Primary outcome was progression-free survival (PFS) 4/13
The trial showed a very modest benefit to maintenance pemetrexed, with median PFS increasing from 2.6 to 4.3 months, and median overall survival from 10.6 to 13.4 months. This was exciting at the time, but pales compared to the benefits in more recent TKI and immunotherapy trials 

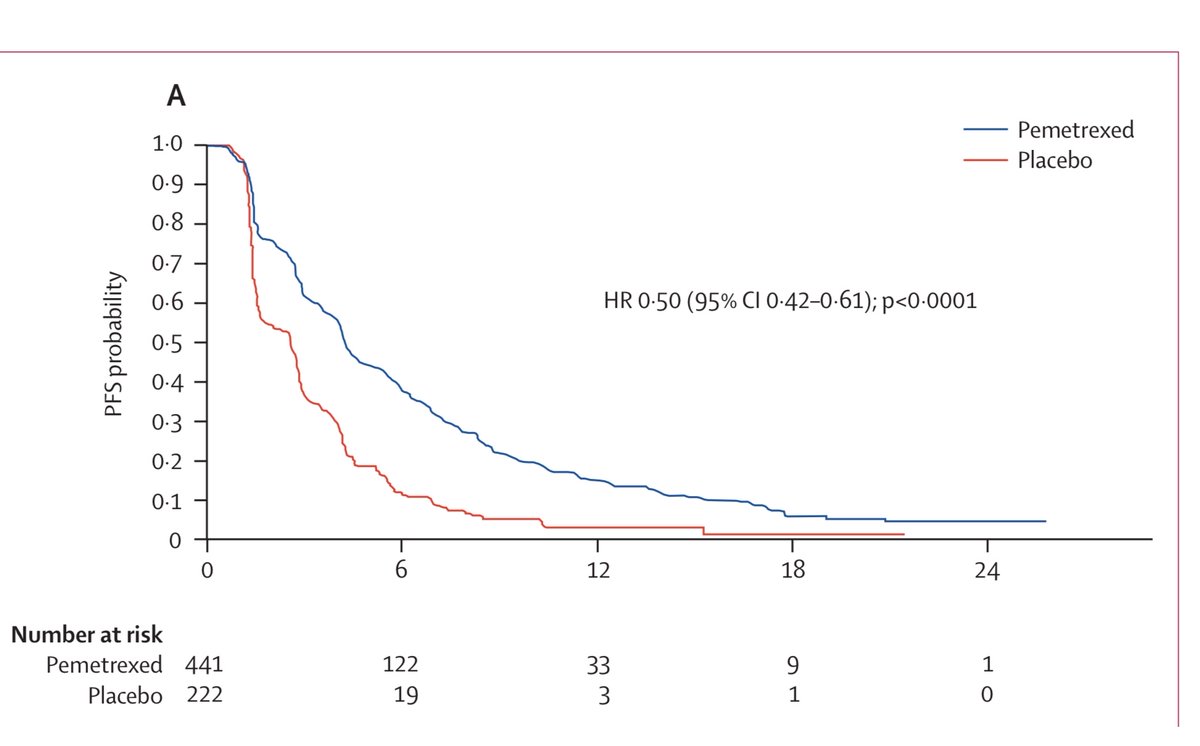

As pemetrexed became an accepted first-line option, a trial called PARAMOUNT was performed that essentially duplicated this trial, but with pemetrexed in the first-line. The results were very similar. 6/13 

Maintenance pemetrexed has become an almost invariable standard of care for non-squamous NSCLC, particularly with the adoption of KN-189 (13 Nov). I still wonder, though, if there are some patients who would benefit more from the treatment break than from continuous chemo. 7/13 



These trials used a placebo injection for those patients not randomized to maintenance pemetrexed. We’ve seen some studies that used placebo, and many that did not. Why do we care, and what goes into this decision? 8/13
Firstly, a placebo notionally allows blinding, keeping both the patient and the investigator uncertain as to which treatment the patient has been assigned. Obviously, placebo alone is only a reasonable choice if the standard of care is not to receive treatment. 9/13
Blinding is important to mitigate investigator’s biases. It’s more important to have if the outcome is subjective, requiring interpretation. Assessing whether a cancer has progressed can be surprisingly subjective. In contrast, determining survival time is fairly bias-free. 10/13
In some situations, such as drugs with notable side effects, it may not be feasible to have a convincing placebo. But studies have gone to great lengths to have placebos, including studies with sham surgeries and devices, or “active placebos” with some side effects of the drug.
I’m on the fence as to whether the placebo in these trials blinded anyone or not. Pemetrexed is a well-tolerated chemotherapy, but it’s hard for me to imagine that patients previously exposed to the drug (in PARAMOUNT) would not notice a difference with a saline infusion. 12/13
Tomorrow we’ll check in again with a mesothelioma trial, and see how immunotherapy is altering treatment for that disease. 13/13 

• • •
Missing some Tweet in this thread? You can try to
force a refresh