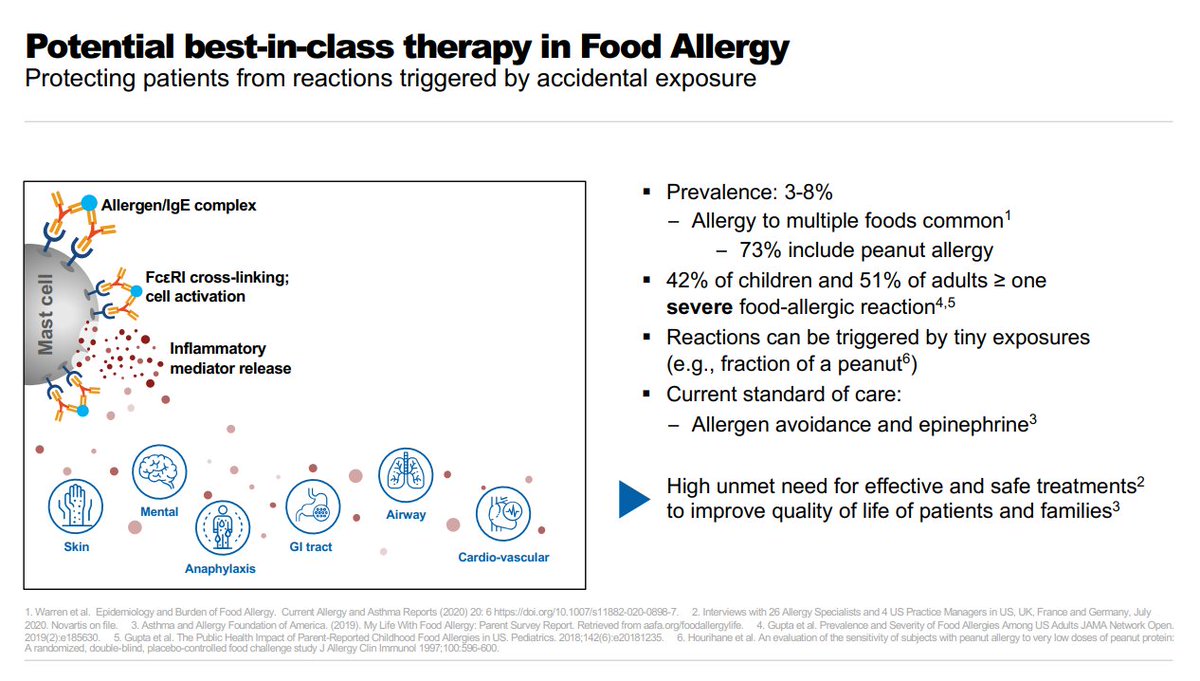
$NVS R&D day slides
novartis.com/sites/novartis…
$SAN $SNY Vaccine day slides from yesterday
Part 1
sanofi.com/-/media/Projec…
Part 2
sanofi.com/-/media/Projec…
novartis.com/sites/novartis…
$SAN $SNY Vaccine day slides from yesterday
Part 1
sanofi.com/-/media/Projec…
Part 2
sanofi.com/-/media/Projec…
$NVS Strategy to 2026
main approvals by mkt potential & current "strength of evidence"
earlier stage pipe in pharmaceuticals & oncology



main approvals by mkt potential & current "strength of evidence"
earlier stage pipe in pharmaceuticals & oncology




only 1 $NVS slide on anti-IL-1B canakinumab in lung cancer, also mentioning gevokizumab backup or in // (different binding mode to IL-1B, more an allosteric modulator than an inhibitor like canak) 

More slides on #KRAS obviously, with JDQ443 anti-KRAS G12C 







$NVS SHP2i TNO155: some PC combo data w/ anti-KRAS showing pot. synergy, pot. interest with other TKIs (EGFR, ALK) 







$NVS SHP2i TNO155 (cont'd): ph1 summary, broad combo program (incl with $MRTX KRAS G12C inh)
Summary of $NVS agents on the KRAS-MAPK
Of note, the recent FIH of YAD/TEAD inh IAG933 (lots of publication on this pathway in past 2/3y or so)


Summary of $NVS agents on the KRAS-MAPK
Of note, the recent FIH of YAD/TEAD inh IAG933 (lots of publication on this pathway in past 2/3y or so)



Now the turn of CAR-Ts w/ $NVS new rapid manuf T-Charge platform, to be featured at #ASH21
<2d manuf (assumedly excl. quality control), aimed to retain naive & stem cell memory T cells in the final soup (same idea than $GRCL Fast-CARs) vs prior process leading to more


<2d manuf (assumedly excl. quality control), aimed to retain naive & stem cell memory T cells in the final soup (same idea than $GRCL Fast-CARs) vs prior process leading to more



$NVS betting on new modalities, also incl. protein degradation with many early stage programs, and through collaborations 







$NVS misc: #radiopharmaceuticals pipeline, collab & pie in GT, approach in RNA tx 







• • •
Missing some Tweet in this thread? You can try to
force a refresh