Nothing conveys so much scientific ignorance so concisely as “Dandelion 22.7pH”
https://twitter.com/BadMedicalTakes/status/1474852953508876291
It’s a common misconception that you can’t have a pH above 14.
You actually CAN have a strong base with pH >14 (or an acid with a pH <0). It’s just super duper hard to dissolve that much base in water.
🧪 Let’s look at what it would take to make a BASIC solution with pH=22!
1/
You actually CAN have a strong base with pH >14 (or an acid with a pH <0). It’s just super duper hard to dissolve that much base in water.
🧪 Let’s look at what it would take to make a BASIC solution with pH=22!
1/
In a chemistry sense, a base is a substance that reacts with an acid.
Water (H₂O) exists as an equilibrium between H+ and -OH:
H₂O ⇌ H⁺ + ⁻OH
In water, a BASE increases the concentration of hydroxide [⁻OH] & decreases the [H⁺]
Now we need a way to measure that!
3/
Water (H₂O) exists as an equilibrium between H+ and -OH:
H₂O ⇌ H⁺ + ⁻OH
In water, a BASE increases the concentration of hydroxide [⁻OH] & decreases the [H⁺]
Now we need a way to measure that!
3/

pH is often taught as a “scale from 0 to 14.” For 99% of uses this simplification works just fine.
But what pH actually measures is the activity of H+ ions, which we approximate with the decimal logarithm of H+ concentration.
pH = log₁₀[H+]
At pH =7, [H+] is 10⁻⁷ mol/L
4/

But what pH actually measures is the activity of H+ ions, which we approximate with the decimal logarithm of H+ concentration.
pH = log₁₀[H+]
At pH =7, [H+] is 10⁻⁷ mol/L
4/
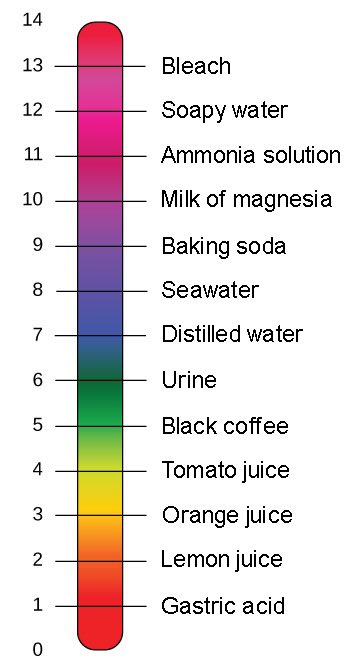

Notice that at a pH of 7, the concentration of ⁻OH is also 10⁻⁷ mol/L.
Because at pH =7, the [H⁺] = [⁻OH], the pH is the same as the pOH. This is why pH=7 is “neutral”; its neither acidic nor basic because there’s the same amount of acid [H⁺] & base [⁻OH] present
3/
Because at pH =7, the [H⁺] = [⁻OH], the pH is the same as the pOH. This is why pH=7 is “neutral”; its neither acidic nor basic because there’s the same amount of acid [H⁺] & base [⁻OH] present
3/
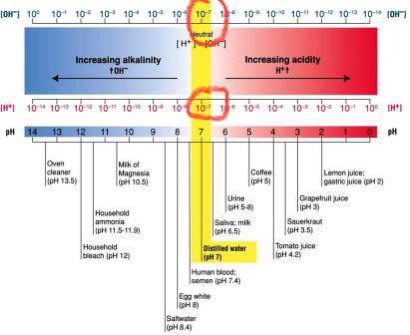
Quick sidebar: people have said that pH “isn’t metric”.
I can see why it’s confusing - a “scale from 0 to 14, where 7 is neutral” seems arbitrary (like water freezing at 32°F & boiling at 212°F).
But the unit we use to calculate pH is mol, which is about as SI as it gets!
4/
I can see why it’s confusing - a “scale from 0 to 14, where 7 is neutral” seems arbitrary (like water freezing at 32°F & boiling at 212°F).
But the unit we use to calculate pH is mol, which is about as SI as it gets!
4/

Another sidebar: at my college, there was a 14 floor science library. As a tribute to pH, each floor was colored according to litmus paper for that pH.
Ugly brutalist architecture but a cool little design touch eh?
5/



Ugly brutalist architecture but a cool little design touch eh?
5/




Back to making crazy basic solutions:
If we want to raise the pH of of water (make it more basic), we need to add a base.
A strong base typically releases ⁻OH & can pull protons (H⁺) off of water. This decreases the amount of [H⁺] & raises the pH.
6/
If we want to raise the pH of of water (make it more basic), we need to add a base.
A strong base typically releases ⁻OH & can pull protons (H⁺) off of water. This decreases the amount of [H⁺] & raises the pH.
6/

If we add 100g of sodium hydroxide (NaOH) to water, this will raise the pH a lot! But how much?
(Definitely don’t try this at 🏠)
NaOH molar mass is 40 g/mol
So 100g of NaOH /(40 g/mol) = 2.5 mol NaOH
So if we dissolve 100gm in 1 L of water we = 2.5 M solution.
7/


(Definitely don’t try this at 🏠)
NaOH molar mass is 40 g/mol
So 100g of NaOH /(40 g/mol) = 2.5 mol NaOH
So if we dissolve 100gm in 1 L of water we = 2.5 M solution.
7/



NaOH is a strong base so it dissociates completely, therefore [OH-] = 2.5 M
Now we can plug that in & calculate the pH
pH = 14 - pOH
-log(2.5M OH) = -0.40
So pH = 14 - (-0.40)
pH = 14.40
👀 We just made a solution with pH >14.
8/
Now we can plug that in & calculate the pH
pH = 14 - pOH
-log(2.5M OH) = -0.40
So pH = 14 - (-0.40)
pH = 14.40
👀 We just made a solution with pH >14.
8/
We did it. We made a solution with a pH of 14.4.
If we use that 14 floor science library analogy we’d be floating about half a floor above the roof!
It’s also super caustic & dangerous. ⚠️🧪
Seriously don’t try this.
So we got >14. But how high can we make the pH go?
9/
If we use that 14 floor science library analogy we’d be floating about half a floor above the roof!
It’s also super caustic & dangerous. ⚠️🧪
Seriously don’t try this.
So we got >14. But how high can we make the pH go?
9/

Can we get the pH higher by adding more NaOH? Hell Yes!
The solubility of NaOH in water is 1000 gm/L.
So instead of dissolving 100gm and making a 2.5M solution we can dissolve 1000gm and make at 25M solution. 😱
-Log₁₀(25) = -1.4
pH = 14 - (-1.4)
pH = 15.4
A pH of 15!
10/


The solubility of NaOH in water is 1000 gm/L.
So instead of dissolving 100gm and making a 2.5M solution we can dissolve 1000gm and make at 25M solution. 😱
-Log₁₀(25) = -1.4
pH = 14 - (-1.4)
pH = 15.4
A pH of 15!
10/



We got to a pH of 15.4!
Using our highly precise science library analogy we are now hovering well above the roof.
But we are still well short of the dandelions putative pH=22.
11/
Using our highly precise science library analogy we are now hovering well above the roof.
But we are still well short of the dandelions putative pH=22.
11/

While we are making 25M NaOH, we are gonna run into a couple problems:
First even if it’s *theoretically* possible it’s gonna take *forever* to dissolve this much NaOH in water. Get used to watching this turn
Oh and it’s probably gonna melt the PTFE stir bar.
12/
First even if it’s *theoretically* possible it’s gonna take *forever* to dissolve this much NaOH in water. Get used to watching this turn
Oh and it’s probably gonna melt the PTFE stir bar.
12/
The second issue is solvation.
Whenever a solute dissolves it becomes surrounded by solvent.
For simplicity we assume that there are far more water molecules than solute. This assumption doesn’t really hold with 25M NaOH.
(The molarity of water itself is 55M)
13/
Whenever a solute dissolves it becomes surrounded by solvent.
For simplicity we assume that there are far more water molecules than solute. This assumption doesn’t really hold with 25M NaOH.
(The molarity of water itself is 55M)
13/

Bottom line it’s gonna be really really difficult to get the pH much higher than 15 in water.
So should we accept defeat and let the dandelion win? Never!
We are going to need a better solvent
14/
So should we accept defeat and let the dandelion win? Never!
We are going to need a better solvent
14/

But what solvent should we use? How does pH even work in solvents other than water?
Turns out it’s *complicated* but a unified pH scale was described in 2010 that we can use to help us find the right one!
onlinelibrary.wiley.com/doi/abs/10.100…
15/

Turns out it’s *complicated* but a unified pH scale was described in 2010 that we can use to help us find the right one!
onlinelibrary.wiley.com/doi/abs/10.100…
15/


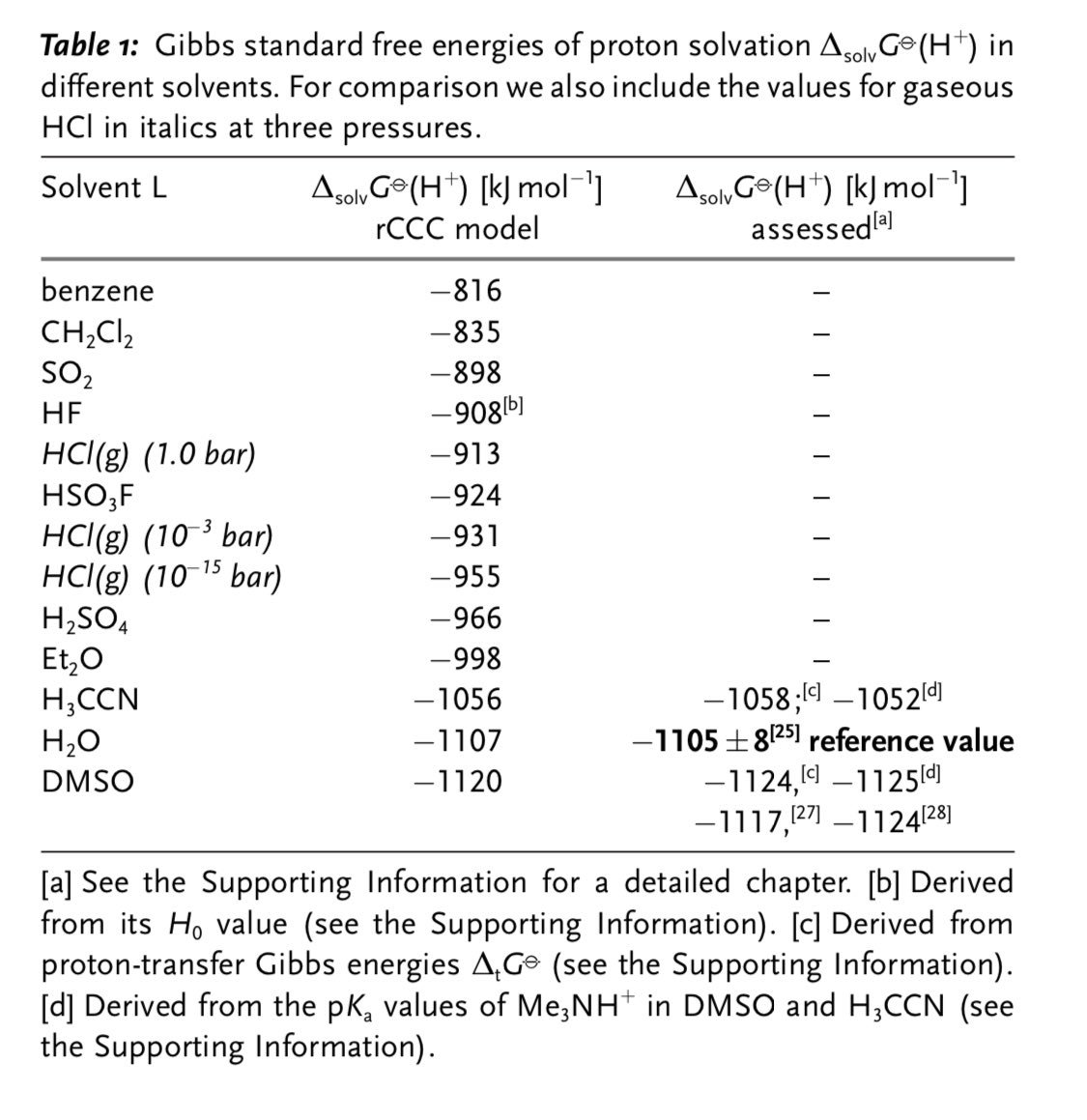
This paper is brilliant btw. Using that original definition of an acid (proton activity) it provides a framework for comparing pH across different solvents.
So now we just need to find the perfect solvent to make a super basic solution to defeat the dandelion!
16/

So now we just need to find the perfect solvent to make a super basic solution to defeat the dandelion!
16/

• • •
Missing some Tweet in this thread? You can try to
force a refresh