#COVID19AB Non-Medical Masks
Misrepresented as Medical Masks
From the outset, the Hinshaw/Lagrange Plan knowingly distributes Non-Medical Masks of such poor quality:
• Manufacturer does not recommend > 4 hours use
• Students must be issued 2 per day
alberta.ca/k-12-learning-…



Misrepresented as Medical Masks
From the outset, the Hinshaw/Lagrange Plan knowingly distributes Non-Medical Masks of such poor quality:
• Manufacturer does not recommend > 4 hours use
• Students must be issued 2 per day
alberta.ca/k-12-learning-…




@SOSAlberta @schill_dawg @gilmcgowan @GosiaGasperoPhD @PopAlberta @jvipondmd @demandsbetter @TehseenLadha @BarryHunt008 @Lauren_Global Distributing Non-Medical Masks is completely counter to @CMOH_Alberta & Education Min @AdrianaLaGrange assertions that the "safe and healthy" return to in-person learning would provide Medical Masks.
alberta.ca/release.cfm?xI…
@wingkarli @amandalhu @UbakaOgbogu @shoffmanAB


alberta.ca/release.cfm?xI…
@wingkarli @amandalhu @UbakaOgbogu @shoffmanAB



@SOSAlberta @schill_dawg @gilmcgowan @GosiaGasperoPhD @PopAlberta @jvipondmd @demandsbetter @TehseenLadha @BarryHunt008 @Lauren_Global @CMOH_Alberta @AdrianaLaGrange @wingkarli @amandalhu @UbakaOgbogu @shoffmanAB @plasercalgary @kasza_leslie @MikeLeskow @teachtheteecher @Mrhockey1231 @Lorian_H @Dave_Khan @Adam_Toy @JuliaWongCBC @RachelNotley When these falsely purported "Medical Masks" finally started arriving mid-week for children who had started Mon 10 Jan, parents and teachers have been sharing pictures asking about them.
I investigated, and here is my response.
I investigated, and here is my response.
https://twitter.com/ZiadFazel/status/1481709530429808641?s=20
Minister @AdrianaLaGrange claims "A medical-grade mask has been tested and meets international standards."
Let's see:
• Requirements definition & procurement spec
• Test results from independent lab (?) to those standards
• Assessment matrix of all options investigated
Let's see:
• Requirements definition & procurement spec
• Test results from independent lab (?) to those standards
• Assessment matrix of all options investigated
@AdrianaLaGrange Except for two specific "Surgical Face Masks", BYD admits their masks are non-medical, and do NOT meet the International Standards that define a Medical Mask.
These are the Standards Health Canada applies, and GoA USED TO respect Before Vanch (BV).
byd.care/pages/faq


These are the Standards Health Canada applies, and GoA USED TO respect Before Vanch (BV).
byd.care/pages/faq



I am concerned about the masks that teachers & other adult staff are getting:
"starting with a 2-week supply of masks (2 masks per day, 20 masks total) for each student and staff member."
Are adults also getting garbage that doesn't last 4 hours?
alberta.ca/k-12-learning-…
"starting with a 2-week supply of masks (2 masks per day, 20 masks total) for each student and staff member."
Are adults also getting garbage that doesn't last 4 hours?
alberta.ca/k-12-learning-…
When I looked into Vanch Masks in April 2020, Health Canada could not investigate because it wasn't the Manufacturer/Exporter making false claims. They'd disclosed their failure to meet Medical Mask standards.
It was the Buyer (GoA) making false claims.
It was the Buyer (GoA) making false claims.
https://twitter.com/ZiadFazel/status/1484369433891987461?s=20
IFR Chlorine Mask fiasco is good precedent by which to judge Minister LaGrange's actions now:
• her staff all over it, especially a DM w TERRIBLE amnesia
• her only recollection: "high quality..right sizing..on time"
• No Transparency/Accountability
ethicscommissioner.ab.ca/media/2809/lag…



• her staff all over it, especially a DM w TERRIBLE amnesia
• her only recollection: "high quality..right sizing..on time"
• No Transparency/Accountability
ethicscommissioner.ab.ca/media/2809/lag…




@sameo416 @gilmcgowan @schill_dawg @RachelNotley @shoffmanAB @KathleenGanley @Lorian_H @jvipondmd @BarryHunt008 @CAPPEM2 I think about what I, as a Professional Engineer, would do here.
About 20 yrs ago, I was hired by Alberta Research Council as a team lead for a Direct Methanol Fuel Cell joint venture with a startup. It quickly became obvious as a scam, but the person most vested was my VP.
About 20 yrs ago, I was hired by Alberta Research Council as a team lead for a Direct Methanol Fuel Cell joint venture with a startup. It quickly became obvious as a scam, but the person most vested was my VP.
@sameo416 @gilmcgowan @schill_dawg @RachelNotley @shoffmanAB @KathleenGanley @Lorian_H @jvipondmd @BarryHunt008 @CAPPEM2 I used to report to the VP through a Director, an honest and fine man, who encouraged me to keep sending him reports in writing.
The Director was then Seconded to another program, and I reported directly to the VP. Who told me to never send him anything on this in writing.
The Director was then Seconded to another program, and I reported directly to the VP. Who told me to never send him anything on this in writing.
(I kept doing it anyway, in my monthly reports to him).
The JV startup was running low on cash, and wanted to issue more shares. They put out a bunch of dishonest press releases, and I got sent for media training to tout the project publicly. I would not repeat the BS.
The JV startup was running low on cash, and wanted to issue more shares. They put out a bunch of dishonest press releases, and I got sent for media training to tout the project publicly. I would not repeat the BS.
Media interviews were then scheduled for me to appear with an executive from the JV startup, and I refused. The Technology Commercialization Office, that was funding the whole project, also ran the PR Office and demanded an explanation. My VP told me he would fire me if I did.
I pulled an all-nighter, and sent a full report to both my VP and TCO VP, an hour before they were to meet to review the project.
Of course, my VP fired me. And of course, I reported it to Auditor General & Securities Commission, and I sued successfully for wrongful dismissal.
Of course, my VP fired me. And of course, I reported it to Auditor General & Securities Commission, and I sued successfully for wrongful dismissal.
Huge hassle. VP "got retired"; startup firm died; ARC restructured.
Moral of the story? Professional Engineers HAVE TO resist the organizational behaviour I see with these non-medical masks, to the point of being fired.
That was a $5m project. This is > 700K schoolchildren.
Moral of the story? Professional Engineers HAVE TO resist the organizational behaviour I see with these non-medical masks, to the point of being fired.
That was a $5m project. This is > 700K schoolchildren.
ICYMI, I just wanted to highlight this part of the Ethics Commissioner's investigation of the August 2020 last-minute purchases of masks for the Sep 2020 school year.
Dr Hinshaw is more involved in Alberta Education mask procurement than people think.
ethicscommissioner.ab.ca/media/2809/lag…
Dr Hinshaw is more involved in Alberta Education mask procurement than people think.
ethicscommissioner.ab.ca/media/2809/lag…

I have a copy of T/GDMDMA 0005-2020 Standard applying to BYD Children's Masks that Hinshaw & Lagrange are distributing to Alberta schoolchildren as Medical Masks.
"The filtration efficiency of the mask to non-oily particles should not be less than 80%."
alberta.ca/release.cfm?xI…



"The filtration efficiency of the mask to non-oily particles should not be less than 80%."
alberta.ca/release.cfm?xI…




@PopAlberta @jvipondmd @GosiaGasperoPhD @noelgibney @LeylaDAsadi @demandsbetter @BarryHunt008 @JBuriak @TehseenLadha @FionaMattatall Teachers + other adult school staff: pls provide pictures of packaging (all sides of box) + product for "medical masks" you are receiving.
I'll look into those too. DMs open for anonymous submission.
@gilmcgowan @schill_dawg @shoffmanAB @UbakaOgbogu @Lorian_H @KathleenGanley
I'll look into those too. DMs open for anonymous submission.
@gilmcgowan @schill_dawg @shoffmanAB @UbakaOgbogu @Lorian_H @KathleenGanley
After emails back-and-forth with @BYDCompany - the manufacturer of these FE2411 Non-Medical Single Use Cartoon Pattern Face Masks for Kids, I have filed a formal @HealthCanada Health Product Complaint.
/c @AdrianaLaGrange @CMOH_Alberta @RicMcIver
canada.ca/en/health-cana…



/c @AdrianaLaGrange @CMOH_Alberta @RicMcIver
canada.ca/en/health-cana…




@BYDCompany @HealthCanada @AdrianaLaGrange @CMOH_Alberta @RicMcIver @SOSAlberta @PopAlberta @jvipondmd @LeylaDAsadi @schill_dawg @gilmcgowan @RachelNotley @RajBhardwajMD @BarryHunt008 @sameo416 This goes beyond the false claims these masks are medical-grade masks, although that is enough grounds for a complaint.
The GoA is acting as distributor, maybe also importer, of these masks. It reasonably ought to know this is a false claim.
@UbakaOgbogu @Lorian_H @Dave_Khan
The GoA is acting as distributor, maybe also importer, of these masks. It reasonably ought to know this is a false claim.
@UbakaOgbogu @Lorian_H @Dave_Khan
I have more serious concerns related to standards compliance and 4-hour exposure limit. @BYDCompany would not release ANY test results or reports to me, even after I explained millions were being distributed to schoolchildren to be worn twice/day.
Sharing portions of emails.


Sharing portions of emails.



Let's get this "international standards" 🐴💩 out of the way.
GDMDMA = Guangdong Medical Device Management Association. It is a voluntary industry association in Guangdong Province, an industrial province in SE China.
It is neither a national nor an international regulator.


GDMDMA = Guangdong Medical Device Management Association. It is a voluntary industry association in Guangdong Province, an industrial province in SE China.
It is neither a national nor an international regulator.



I escalated to the Secretariat for GDMDMA cc: @BYDCompany. Neither responded.
It may just be Window Dressing for "world’s largest PPE face-mask plant..50m masks/day."
Would Microsoft dominate Seattle Area Operating System Developer Association (SAOSDA)?
It may just be Window Dressing for "world’s largest PPE face-mask plant..50m masks/day."
Would Microsoft dominate Seattle Area Operating System Developer Association (SAOSDA)?
@BYDCompany @BYDCompany sued US counterfeiters, with lofty PR.
Yet when @BYDCompany is asked for their own test reports, they resist.
And when told their distributor is sending non-medical masks to children as medical masks, they ignore it.
@GovCanHealth
en.byd.com/news/byd-targe…
Yet when @BYDCompany is asked for their own test reports, they resist.
And when told their distributor is sending non-medical masks to children as medical masks, they ignore it.
@GovCanHealth
en.byd.com/news/byd-targe…
@BYDCompany @GovCanHealth The voluntary industry standard @BYDCompany purports to meet is not difficult. (These masks don't meet Chinese National Standards.)
eg. 80% PFE < 95% PFE required by ASTM F2100.
So when they won't disclose lab reports for their own soft standard.....
codeofchina.com/standard/TGDMD…

eg. 80% PFE < 95% PFE required by ASTM F2100.
So when they won't disclose lab reports for their own soft standard.....
codeofchina.com/standard/TGDMD…


@BYDCompany @GovCanHealth Not just to me that @BYDCompany will not release test reports.
They + their Canadian subsidiary also sell this mask which purports to meet Chinese YY/T 0969-2013.
You may recall AB Health claimed it was sorta equivalent to ASTM F2100 for the Vanch 🐶💩
bydcare.ca/single-use-fac…
They + their Canadian subsidiary also sell this mask which purports to meet Chinese YY/T 0969-2013.
You may recall AB Health claimed it was sorta equivalent to ASTM F2100 for the Vanch 🐶💩
bydcare.ca/single-use-fac…

What's this "Australia TGA ARTG 332299" also listed under Standard?
Australian Regulatory Approval?
NO.
That's from their Therapeutic Goods Administration (TGA) "Post-market review of face masks."
Same thing I am triggering w @GovCanHealth Complaint.
tga.gov.au/post-market-re…
Australian Regulatory Approval?
NO.
That's from their Therapeutic Goods Administration (TGA) "Post-market review of face masks."
Same thing I am triggering w @GovCanHealth Complaint.
tga.gov.au/post-market-re…
I looked deeper.
332299 is an amateur mistake: Surgical mask does not meet fluid resistance near the embossed logo.
Easy to solve: patient signs a pre-op contract promising not to bleed anywhere near the logos of anyone in the OR or post-op recovery unit.
@AB_MD_WarRoom
332299 is an amateur mistake: Surgical mask does not meet fluid resistance near the embossed logo.
Easy to solve: patient signs a pre-op contract promising not to bleed anywhere near the logos of anyone in the OR or post-op recovery unit.
@AB_MD_WarRoom

ARTG 337182 is bigger:
• Importer "Trustee for DKT Family Trust" (Australian wholesaler)
• Did not provide compliance, safety, performance, quality... relevant supply information, including list of customers to whom they may have been distributed.
apps.tga.gov.au/Prod/sara/arn-…



• Importer "Trustee for DKT Family Trust" (Australian wholesaler)
• Did not provide compliance, safety, performance, quality... relevant supply information, including list of customers to whom they may have been distributed.
apps.tga.gov.au/Prod/sara/arn-…




Australia is kinda an important market to a Chinese mask manufacturer.
Yet @BYDCompany importer did not provide needed disclosure to 🇦🇺 regulator, so had their surgical mask kicked out of medical use.
IIRC, AB Health claimed that YY/T 0969 Std was fine for Vanch.
Yet @BYDCompany importer did not provide needed disclosure to 🇦🇺 regulator, so had their surgical mask kicked out of medical use.
IIRC, AB Health claimed that YY/T 0969 Std was fine for Vanch.
@BYDCompany Time to wrap up. Brain tired = GIF parade.
Where were we with BYD's cartoon masks?
✅false claims by GoA's cartoon leaders the masks are "medical-grade"
✅resistance by BYD to disclose the lab test reports they confirmed they have
🔲 concerns about exposure limits for kids


Where were we with BYD's cartoon masks?
✅false claims by GoA's cartoon leaders the masks are "medical-grade"
✅resistance by BYD to disclose the lab test reports they confirmed they have
🔲 concerns about exposure limits for kids



@BYDCompany I don't buy BYD's explanation. I looked for WHO guidance they said it "seems to come from":
who.int/news-room/ques…
Nothing specific about 4 hours. That seems to be an exposure limit from biocompability testing. I need the depth of eg. @JBuriak @KevinHedges15 @JenniferKShea
who.int/news-room/ques…
Nothing specific about 4 hours. That seems to be an exposure limit from biocompability testing. I need the depth of eg. @JBuriak @KevinHedges15 @JenniferKShea

But I have no data to share with these experts:
• except my correspondence with @BYDCompany who will not give it to me
• their 🇦🇺importer apparently won't do that with their regulator
• they do not have regulatory approval in USA or Canada where we can maybe review files
• except my correspondence with @BYDCompany who will not give it to me
• their 🇦🇺importer apparently won't do that with their regulator
• they do not have regulatory approval in USA or Canada where we can maybe review files
@BYDCompany On masks for children: "WHO advises that people always consult and abide by local authorities on recommended practices in their area."
Ha! That would be @CMOH_Alberta and Education Minister @AdrianaLaGrange who are distributing these non-medical masks as "medical grade".
Ha! That would be @CMOH_Alberta and Education Minister @AdrianaLaGrange who are distributing these non-medical masks as "medical grade".
I am not an expert @JBuriak @KevinHedges15 @JenniferKShea or paediatrician @TehseenLadha or @DrPeterNieman.
ncbi.nlm.nih.gov/pmc/articles/P…
But I sense risk in my shallow research of specific 4-hr limits that force GoA to buy 2 masks/day for kids, which they falsely claim are medical.

ncbi.nlm.nih.gov/pmc/articles/P…
But I sense risk in my shallow research of specific 4-hr limits that force GoA to buy 2 masks/day for kids, which they falsely claim are medical.


@JBuriak @KevinHedges15 @JenniferKShea @TehseenLadha @DrPeterNieman I combined:
• GoA's misrepresentations these masks are medical grade
• GoA history w IFR Chlorine Masks & Vanch Masks for HCW
• BYD's resistance to disclosing their lab tests & questionable behaviour w national regulators
• my Spidey Senses
And I saw too much risk.
• GoA's misrepresentations these masks are medical grade
• GoA history w IFR Chlorine Masks & Vanch Masks for HCW
• BYD's resistance to disclosing their lab tests & questionable behaviour w national regulators
• my Spidey Senses
And I saw too much risk.
Good night all.
This has been bothering me for two weeks, and I spent all afternoon on @GovCanHealth formal complaint, after little bits of work here & there in the evening this week.
I learned from the Vanch masks experience, but am open to polite advice if you have it.
This has been bothering me for two weeks, and I spent all afternoon on @GovCanHealth formal complaint, after little bits of work here & there in the evening this week.
I learned from the Vanch masks experience, but am open to polite advice if you have it.
At 5.05am, @GovCanHealth Central Triage Unit forwarded my complaint to Medical Devices section for consideration.
@CMOH_Alberta + @AdrianaLaGrange claim; @RicMcIver distributes:
"A medical-grade mask has been tested and meets international standards."
alberta.ca/release.cfm?xI…



@CMOH_Alberta + @AdrianaLaGrange claim; @RicMcIver distributes:
"A medical-grade mask has been tested and meets international standards."
alberta.ca/release.cfm?xI…




@GovCanHealth @CMOH_Alberta @AdrianaLaGrange @RicMcIver @SOSAlberta @PopAlberta @TehseenLadha @jvipondmd @demandsbetter @RajBhardwajMD @GosiaGasperoPhD @sameo416 @LeylaDAsadi @Lauren_Global I have also written @BYDCompany Canadian operations, located at ALCOS Machinery Inc plant in Newmarket, ON.
bydcare.ca/contact-us
Canadian retail partner @SciInnovation17
sciinnovation.ca
GoA distributing 10 masks/week to a pool > 330K schoolchildren through Gr 5.



bydcare.ca/contact-us
Canadian retail partner @SciInnovation17
sciinnovation.ca
GoA distributing 10 masks/week to a pool > 330K schoolchildren through Gr 5.




Red Flag: Still no lab test reports.
I've been writing BYD Care global HQ in California, who list these masks on their website.
Cdn Sales told me they are out of the mask business.
Today BYD's exclusive PPE distributor told me they didn't supply AB Education. No test reports.



I've been writing BYD Care global HQ in California, who list these masks on their website.
Cdn Sales told me they are out of the mask business.
Today BYD's exclusive PPE distributor told me they didn't supply AB Education. No test reports.



@SOSAlberta @LeylaDAsadi @BarryHunt008 @JenniferKShea @GovCanHealth @KevinHedges15 @jvipondmd @hardeepr33 @GosiaGasperoPhD @Lauren_Global To match Minister's total of 65.6m masks:
• assign two (2) masks per person per day
• for children AND adults 🧐
• students, teachers, ed assistants, CUPE staff..
• still add 19K DAILY users.
Some intermediary/crony making out like a masked bandit.


• assign two (2) masks per person per day
• for children AND adults 🧐
• students, teachers, ed assistants, CUPE staff..
• still add 19K DAILY users.
Some intermediary/crony making out like a masked bandit.
https://twitter.com/AdrianaLaGrange/status/1484632449602572289?s=20&t=0naRkl4yVSR7kc8WbiXUEA


@SOSAlberta @LeylaDAsadi @BarryHunt008 @JenniferKShea @GovCanHealth @KevinHedges15 @jvipondmd @hardeepr33 @GosiaGasperoPhD @Lauren_Global @schill_dawg @gilmcgowan @KathleenGanley @Lorian_H @DASouthe What parent would buy 4-hr masks for their kids?
"Honey, wear this from 8am, then take the other out of your backpack at Noon to wear until 4pm. Have fun in Grade 1. xoxo"
@AdrianaLaGrange bought this crap, with our $$, then dumped it on teachers to make sure the kids change.
"Honey, wear this from 8am, then take the other out of your backpack at Noon to wear until 4pm. Have fun in Grade 1. xoxo"
@AdrianaLaGrange bought this crap, with our $$, then dumped it on teachers to make sure the kids change.
In Nov 2021, @CPHO_Canada recommended respirators & medical masks, and deprecated non-medical masks.
That made this inventory unsellable.♻️
Instead GoA bought 65.6m, lied they are medical-grade, make our kids breathe through them for 4 hours, then 🗑️.



That made this inventory unsellable.♻️
Instead GoA bought 65.6m, lied they are medical-grade, make our kids breathe through them for 4 hours, then 🗑️.
https://twitter.com/cpho_canada/status/1459579402832994313



Hi all. I need your help identifying all the masks AB Education is sending from ECS - Grade 12, Teachers & Staff.
Please tweet or DM:
• photos of masks and packaging
• which grade.
BYD makes FE2411 in cartoon & plain. No cartoon does not mean safe.
byd.care/collections/all
Please tweet or DM:
• photos of masks and packaging
• which grade.
BYD makes FE2411 in cartoon & plain. No cartoon does not mean safe.
byd.care/collections/all

@SOSAlberta @schill_dawg @gilmcgowan @TehseenLadha @DrPeterNieman @LeylaDAsadi @GosiaGasperoPhD @PopAlberta @caitlynsmitman @LukaszukAB On Friday, @GovCanHealth confirmed they are investigating my complaint about AB Education distributing these non-medical masks as "medical-grade", and invited me to send them more information.
Please help me protect your children and the adults working in schools. DMs open.

Please help me protect your children and the adults working in schools. DMs open.


Another issue: Gr 6 teacher sent me pic of Medicom SafeMask® Premier Pediatric Earloop.
@MedicomGlobal claims ASTM Level 1 - Class 1 Medical Device.
But @GovCanHealth does not show "SafeMask Premier" or "Model 2038" as authorized Class 1 Medical Device.
medicom.com/en_ca/product/…



@MedicomGlobal claims ASTM Level 1 - Class 1 Medical Device.
But @GovCanHealth does not show "SafeMask Premier" or "Model 2038" as authorized Class 1 Medical Device.
medicom.com/en_ca/product/…




@MedicomGlobal @GovCanHealth @hardeepr33 @SOSAlberta @TehseenLadha @DrPeterNieman @PopAlberta @jvipondmd @GosiaGasperoPhD @BarryHunt008 @MikeLeskow @caitlynsmitman Good News on Medicom Masks.
Tx @MedicomGlobal for your helpful DM, which I verified independently yesterday.
Class I Medical Devices like masks do not require their own authorization if manufacturer already holds a Medical Device Establishment License.
canada.ca/en/health-cana…



Tx @MedicomGlobal for your helpful DM, which I verified independently yesterday.
Class I Medical Devices like masks do not require their own authorization if manufacturer already holds a Medical Device Establishment License.
canada.ca/en/health-cana…


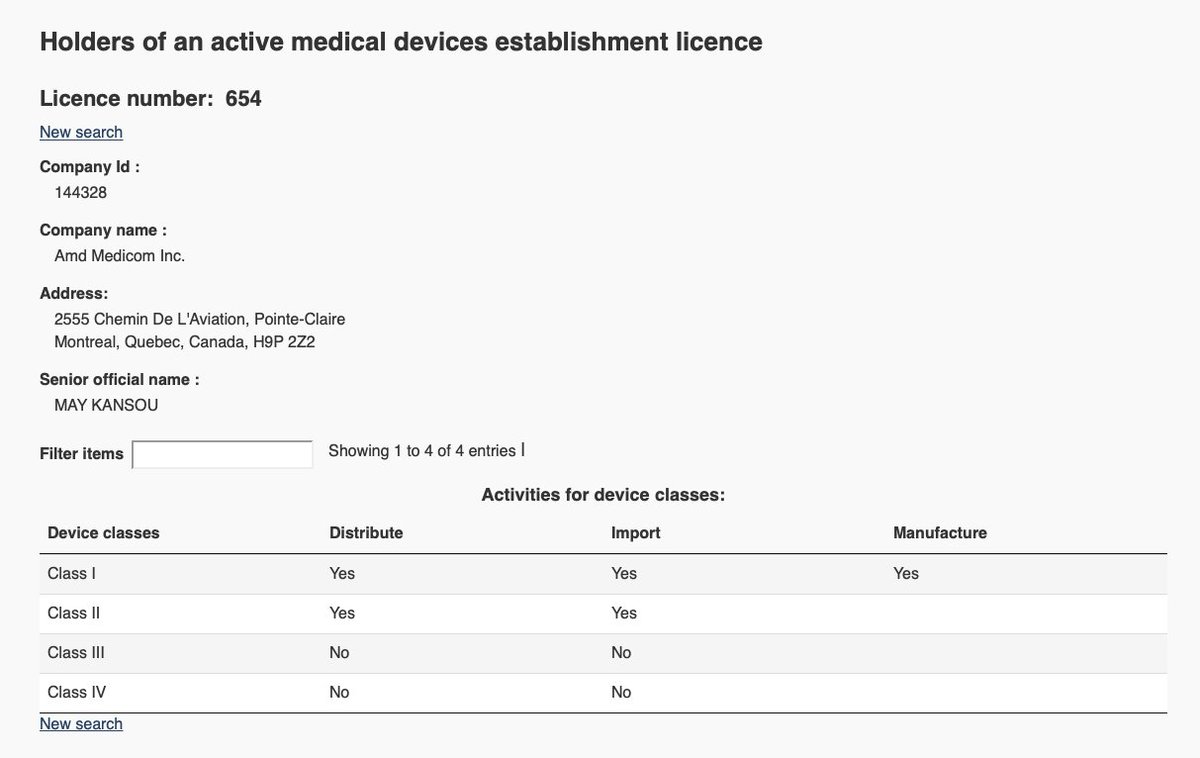

In stark contrast to @BYDCompany covering up the tests forcing its 4-hour exposure limit, Medicom provides a Safety Data Sheet (SDS) in the Technical Information for its product.
I defer to @KevinHedges15 @JenniferKShea @JBuriak on its completeness.
medicom.com/en_ca/product/…


I defer to @KevinHedges15 @JenniferKShea @JBuriak on its completeness.
medicom.com/en_ca/product/…



Anyone notice:
• until Fri 4 Feb, when @GovCanHealth acted on my complaint
• @CMOH_Alberta & Education Min @AdrianaLaGrange were still distributing 65.6m masks through @RicMcIver POC over 8 weeks
• using obviously false claim these are "medical-grade" masks for "added safety"
• until Fri 4 Feb, when @GovCanHealth acted on my complaint
• @CMOH_Alberta & Education Min @AdrianaLaGrange were still distributing 65.6m masks through @RicMcIver POC over 8 weeks
• using obviously false claim these are "medical-grade" masks for "added safety"

@GovCanHealth @CMOH_Alberta @AdrianaLaGrange @RicMcIver @PopAlberta @jvipondmd @GosiaGasperoPhD @hardeepr33 @LeylaDAsadi @RajBhardwajMD @TehseenLadha @Lorian_H @demandsbetter @caitlynsmitman But yesterday:
• when Premier @jkenney removal of public health measures made Personal Protective Equipment more important
• @AdrianaLaGrange suddenly found "having the ability to be animated & joyful" and "mental health impacts" even more important.
• when Premier @jkenney removal of public health measures made Personal Protective Equipment more important
• @AdrianaLaGrange suddenly found "having the ability to be animated & joyful" and "mental health impacts" even more important.
https://twitter.com/AdrianaLaGrange/status/1491212799611002881?s=20&t=-TzwKiXwi01f5eykl3PIAw
I'd like to show a deficiency I see with this type of media coverage given GoA's habit of gaslighting.
I'm not criticizing anyone personally, or media in general, so I am glad this @ctvedmonton is not credited to a specific reporter.
edmonton.ctvnews.ca/experts-questi…
I'm not criticizing anyone personally, or media in general, so I am glad this @ctvedmonton is not credited to a specific reporter.
edmonton.ctvnews.ca/experts-questi…
The article had 3 non-government experts who I have worked with/am working with, and one government statement which is demonstrably false.
I get that reporters are getting official statements on the record, which has lasting value, as well as giving the public fair balance.
I get that reporters are getting official statements on the record, which has lasting value, as well as giving the public fair balance.

But GoA statement was published on all channels with no sign of pushback. GoA is:
• distributing millions of masks to schools
• claiming they are medical-grade + tested to meet international standards
So I would expect an onus on them to provide more than a sound bite.
• distributing millions of masks to schools
• claiming they are medical-grade + tested to meet international standards
So I would expect an onus on them to provide more than a sound bite.

This "98% as effective as N95" claim is obvious 🐴💩, and any of the 3 experts interviewed in the story could have provided evidence to the contrary.
So I would at least like to see "We followed up, asking about A,B and C but as of press time did not receive a response."
So I would at least like to see "We followed up, asking about A,B and C but as of press time did not receive a response."
The pattern:
• experts/parent/advocate with "passion" raise concerns
• government provides deceptive soundbite
• no sign of pushback/followup before story published
With a chronically deceptive government, and very little investigative reporting, there has to be better way.
• experts/parent/advocate with "passion" raise concerns
• government provides deceptive soundbite
• no sign of pushback/followup before story published
With a chronically deceptive government, and very little investigative reporting, there has to be better way.
CTV story was published 2 Jan, when we only knew GoA stated intent, not about the BYD masks so non-medical they have 4-hr exposure limit.
I became investigator & complained to regulator.
But the reporters I am speaking with now won't ask GoA for more than their sound bite.
I became investigator & complained to regulator.
But the reporters I am speaking with now won't ask GoA for more than their sound bite.
Speaking only for myself, I saw @PopAlberta, @jvipondmd, @GosiaGasperoPhD, @NeejaB, @TehseenLadha, @UbakaOgbogu, @Lorian_H et al give generous, expert explanations.
Grossly disproportionate to the deceptive soundbites GoA gets away with.
I don't know how to fix this.
Grossly disproportionate to the deceptive soundbites GoA gets away with.
I don't know how to fix this.
@PopAlberta @jvipondmd @GosiaGasperoPhD @NeejaB @TehseenLadha @UbakaOgbogu @Lorian_H On 21 Jan, this Minister was 16m "medical-grade" masks into CMOH-approved 8-wk plan to distribute 65.6m masks into AB schools, for children as young as 6 to wear.
Now she's denigrating teachers that, until 5pm yesterday, were under her order to do that.

Now she's denigrating teachers that, until 5pm yesterday, were under her order to do that.
https://twitter.com/AdrianaLaGrange/status/1491564924493041670?s=20&t=U--sVi3TxdnwoF9IcZbs2g

@PopAlberta @jvipondmd @GosiaGasperoPhD @NeejaB @TehseenLadha @UbakaOgbogu @Lorian_H @schill_dawg @gilmcgowan @shoffmanAB @RajBhardwajMD @caitlynsmitman @Lauren_Global Over last month, I have received many photos, and boxes of masks, from parents & schools.
This is a typical distribution. Boxes of 50 masks are opened by school office staff, repacked into bags of 20, and given to students.
Sometimes the teachers see the box; usually not.



This is a typical distribution. Boxes of 50 masks are opened by school office staff, repacked into bags of 20, and given to students.
Sometimes the teachers see the box; usually not.




@PopAlberta @jvipondmd @GosiaGasperoPhD @NeejaB @TehseenLadha @UbakaOgbogu @Lorian_H @schill_dawg @gilmcgowan @shoffmanAB @RajBhardwajMD @caitlynsmitman @Lauren_Global @SOSAlberta @BarryHunt008 @JenniferKShea @smbilodeau @Adam_Toy @LiveWire_DK @jansplanet In nearly all cases, neither teachers nor parents ever see the boxes the masks came in, identifying the supplier & product, and defining its quality standards.
Which is a serious problem, because it makes parents completely reliant on GoA's false claims eg. "medical-grade".
Which is a serious problem, because it makes parents completely reliant on GoA's false claims eg. "medical-grade".
I've been looking closely at GoA's mask purchases since the Vanch 💩in April 2020, so when I see "Orpyx" embossed into the seam, I know they are:
orpyx.com/ppe
ADULT-SIZE MASKS.
• Going to kids as young as Grade 3
• School boards & charter schools
• 177 x 95 mm
orpyx.com/ppe
ADULT-SIZE MASKS.
• Going to kids as young as Grade 3
• School boards & charter schools
• 177 x 95 mm
The Orpyx masks are the largest I've seen in this distribution:
Adult Blue Poly
• Orpyx 177 x 95
• Make 99 NonMedical 175 x 95 😱
Children's Masks
• BYD FE2411 145 x 95 😱
• Medicom Pediatric Masks 140 x 85
See Medicom's good explanations why children need smaller masks:


Adult Blue Poly
• Orpyx 177 x 95
• Make 99 NonMedical 175 x 95 😱
Children's Masks
• BYD FE2411 145 x 95 😱
• Medicom Pediatric Masks 140 x 85
See Medicom's good explanations why children need smaller masks:

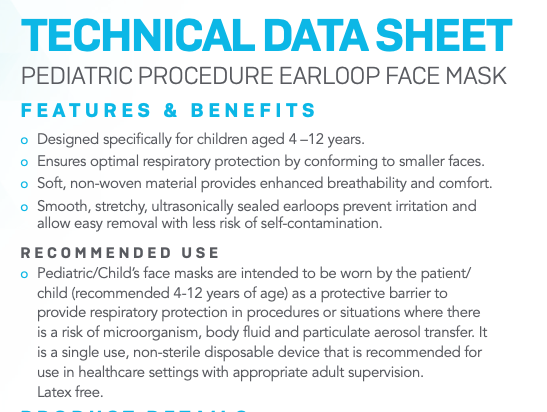

I don't have a concern w regulatory compliance of currently shipping Orpyx. Made in Calgary under an active Class I MDEL.
My concern is AB Education & CMOH distributing them to children too small for the mask.
• Grade 3?! They won't fit 8 & 9-yr-olds at all.
• Grade 6? Maybe.
My concern is AB Education & CMOH distributing them to children too small for the mask.
• Grade 3?! They won't fit 8 & 9-yr-olds at all.
• Grade 6? Maybe.

@JenniferKShea @TehseenLadha @DrPeterNieman @KevinHedges15 @SOSAlberta @caitlynsmitman @jvipondmd @GosiaGasperoPhD @LeylaDAsadi @demandsbetter Here for comparison is an Orpyx 177 x 95 mm Adult Mask, on which I have marked Children's Size 140 x 85 mm.
That 37 mm difference in width works out to about 19 mm further on each side. Good luck keeping the earloops on.
If you did, imagine the gaps around all 4 edges.
That 37 mm difference in width works out to about 19 mm further on each side. Good luck keeping the earloops on.
If you did, imagine the gaps around all 4 edges.

Make 99 NonMedical Masks, being distributed to Grade 6+ (175 x 95 mm).
4 sides of box: “For NonMedical Use”.
No date or lot code anywhere on/in box, in bag, or on masks.
Parents don’t see ANY of this.
Would you buy this for your kids?
@AdrianaLaGrange & @CMOH_Alberta did.
4 sides of box: “For NonMedical Use”.
No date or lot code anywhere on/in box, in bag, or on masks.
Parents don’t see ANY of this.
Would you buy this for your kids?
@AdrianaLaGrange & @CMOH_Alberta did.
Let's dig into Make 99. The QR code on the box led me to their Products Page 😱
make99medical.com/collections/all
Where they apparently sell "Kids ATSM Level 3 Medical Face Masks" [sic]:
• unknown qty or size
• purportedly made in Canada
• no description of materials or construction

make99medical.com/collections/all
Where they apparently sell "Kids ATSM Level 3 Medical Face Masks" [sic]:
• unknown qty or size
• purportedly made in Canada
• no description of materials or construction


They claim to sell adult medical masks. Note the sloppy descriptions:
• 2 masks say "Medical Level" but not which level
• 1 mask just says "Medical"
• 1 mask says "Level III Medical" when the right description should be "Level 3".
Crap supplier. Perfect for your kids, right?
• 2 masks say "Medical Level" but not which level
• 1 mask just says "Medical"
• 1 mask says "Level III Medical" when the right description should be "Level 3".
Crap supplier. Perfect for your kids, right?

Let's look at their best (?) mask
• at least the *website photo* shows individually wrapped
• filtration specs I don't believe
• box says Level 2; website says Level III and 3
• ASTM F2100 Table wrong in several places
make99medical.com/products/medic…



• at least the *website photo* shows individually wrapped
• filtration specs I don't believe
• box says Level 2; website says Level III and 3
• ASTM F2100 Table wrong in several places
make99medical.com/products/medic…




Most expensive $30 masks "specially made to prevent the spread of Omicron & Delta variants".
"Please don’t discount their qualities because of their beauty value. All styles are made at the medical standards."
"ASTM F2100 Standard and passed very strict professional testings."

"Please don’t discount their qualities because of their beauty value. All styles are made at the medical standards."
"ASTM F2100 Standard and passed very strict professional testings."


Box says
"Manufactured By: Make 99
#33 1339 40th Ave NE
Calgary.."
"McCall 40" business park:
hungerfordproperties.com/assets/documen…
Make 99 has ~1600 sq ft main floor; 2200 total.
I guess their "Made in Canada" stuff is made elsewhere in Canada.
Or it may really be imported from China.


"Manufactured By: Make 99
#33 1339 40th Ave NE
Calgary.."
"McCall 40" business park:
hungerfordproperties.com/assets/documen…
Make 99 has ~1600 sq ft main floor; 2200 total.
I guess their "Made in Canada" stuff is made elsewhere in Canada.
Or it may really be imported from China.



Looks like Make 99 printed a box for a certain "individually wrapped" product, but couldn't get it, so they just substituted something blue that came in bulk bags of 50.
I'll be making a new complaint to @GovCanHealth about GoA "Medical Grade" & Make 99.
Tomorrow. Sleep now.
I'll be making a new complaint to @GovCanHealth about GoA "Medical Grade" & Make 99.
Tomorrow. Sleep now.
CMOH & Education Minister: "A medical-grade mask has been tested and meets international standards."
Make 99: "Wash hands thoroughly with soap & water for 1 minute."
Health Canada, CDC: "at least 20 seconds"
So Make 99 EXCEEDS international standards?
alberta.ca/release.cfm?xI…



Make 99: "Wash hands thoroughly with soap & water for 1 minute."
Health Canada, CDC: "at least 20 seconds"
So Make 99 EXCEEDS international standards?
alberta.ca/release.cfm?xI…




In my Make 99 video ⬆️, I suspected GoA is using this "Staying Safe & Healthy" plan to burn off worthless inventory.
GoA pushed out 14m in June 2020 Fast Food Fiasco:
cbc.ca/news/canada/ca…
declared it a success, then pushed 20m more in July.
edmonton.ctvnews.ca/alberta-to-dis…
GoA pushed out 14m in June 2020 Fast Food Fiasco:
cbc.ca/news/canada/ca…
declared it a success, then pushed 20m more in July.
edmonton.ctvnews.ca/alberta-to-dis…
Auditors require GoA/AHS/anyone to write down inventory to lower of cost or market value.
• In 2020/21, the Feds contributed $2b for COVID-19
• AHS spent $900m on supplies, "significant purchase commitments"for PPE, leaving $417m at 31 Mar 2021 yearend
albertahealthservices.ca/assets/about/p…



• In 2020/21, the Feds contributed $2b for COVID-19
• AHS spent $900m on supplies, "significant purchase commitments"for PPE, leaving $417m at 31 Mar 2021 yearend
albertahealthservices.ca/assets/about/p…




Looking to 31 Mar 2022 yearend, there is likely MUCH more PPE inventory, much of it subject to huge write downs.
This plan to burn off 65.6m masks looks like a cynical strategy to minimize that:
• only BYD forces 2/day, not any other mask I've seen
• 19K people fudge factor
This plan to burn off 65.6m masks looks like a cynical strategy to minimize that:
• only BYD forces 2/day, not any other mask I've seen
• 19K people fudge factor

@SOSAlberta @jvipondmd @BarryHunt008 @Lauren_Global @CAPPEM2 @TheBreakdownAB @Adam_Toy @shoffmanAB @joececiyyc @gilmcgowan Ethical people do not avoid inventory write downs by:
• distributing non-medical masks like Make 99
• dangerous masks like BYD FE2411
• for children + school staff to breathe through
• under false claim they are "medical-grade", "tested", and "meet international standards"



• distributing non-medical masks like Make 99
• dangerous masks like BYD FE2411
• for children + school staff to breathe through
• under false claim they are "medical-grade", "tested", and "meet international standards"




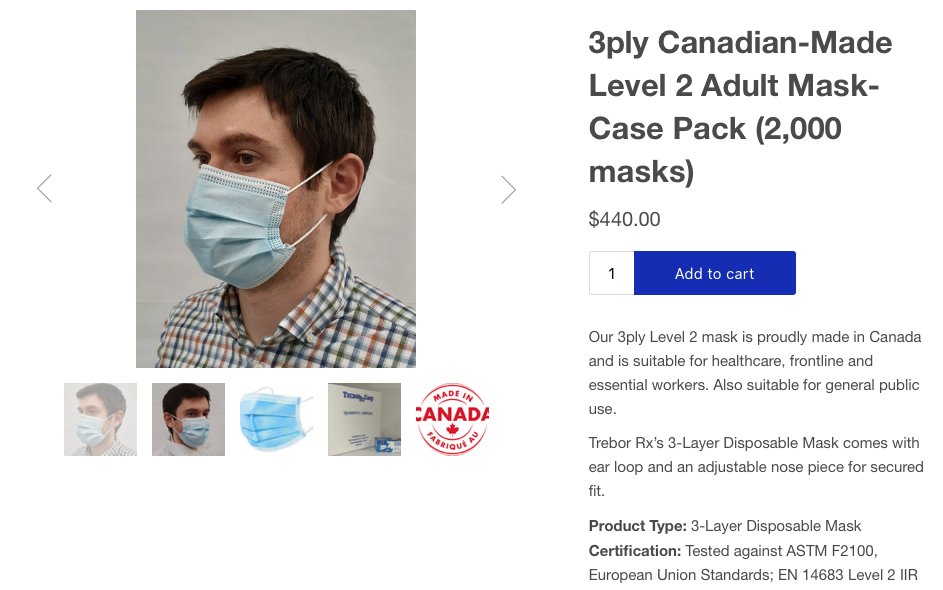
@SOSAlberta @jvipondmd @BarryHunt008 @Lauren_Global @CAPPEM2 @TheBreakdownAB @Adam_Toy @shoffmanAB @joececiyyc @gilmcgowan @Lorian_H @TehseenLadha @DrPeterNieman @picardonhealth @NatashaFatah @CherylMDFRCPC @caitlynsmitman @demandsbetter @schill_dawg @RachelNotley Even Orpyx 3-ply poly ASTM F2100 masks can cause writedowns:
orpyx.com/orpyx-news/mad…
Orpyx: $1.50 each for 40 million masks
Market Value from @CAPPEM2
Trebor RX: $0.22 for 2000
CANADAMASQ: $0.24 for 900
Doesn't let you push Adult masks onto Grade 3 kids.
@UbakaOgbogu


orpyx.com/orpyx-news/mad…
Orpyx: $1.50 each for 40 million masks
Market Value from @CAPPEM2
Trebor RX: $0.22 for 2000
CANADAMASQ: $0.24 for 900
Doesn't let you push Adult masks onto Grade 3 kids.
@UbakaOgbogu


Look at the level of deception from the Premier's Office:
• Notice how the statement makes no mention of biocompatibility or exposure limits?
• It's like GoA knows about the 4-hr limit from BYD
edmonton.ctvnews.ca/experts-questi…
"Medical grade" since 30 Dec:
alberta.ca/release.cfm?xI…

• Notice how the statement makes no mention of biocompatibility or exposure limits?
• It's like GoA knows about the 4-hr limit from BYD
edmonton.ctvnews.ca/experts-questi…
"Medical grade" since 30 Dec:
alberta.ca/release.cfm?xI…


“..when properly fitted are 98% as effective as N95 masks.”
tandfonline.com/doi/full/10.10…
Properly fitted = using a Mask Fitter on top of the medical mask.
See column graph:
• grey - no fitter
• red - Badger Seal
• blue - Fix The Mask
GoA did NOT provide any mask fitters.



tandfonline.com/doi/full/10.10…
Properly fitted = using a Mask Fitter on top of the medical mask.
See column graph:
• grey - no fitter
• red - Badger Seal
• blue - Fix The Mask
GoA did NOT provide any mask fitters.




As I showed above, the BYD masks are *supposed to meet* their voluntary industry standard T/GDMDMA 0005-2020.
But it only has 80% particle filtration efficiency.
So even if you duct-taped the edges of the mask to the kid's face, you only get 80%, not the 98%+ of an N95.



But it only has 80% particle filtration efficiency.
So even if you duct-taped the edges of the mask to the kid's face, you only get 80%, not the 98%+ of an N95.



@TehseenLadha @jvipondmd @GosiaGasperoPhD @BarryHunt008 @LeylaDAsadi @CAPPEM2 @demandsbetter @DrPeterNieman @Lauren_Global @Adam_Toy That's just poor fit & filtration.
My BIGGEST concern with these BYD masks is 4-hour exposure limit.
They lied to me when I asked for an explanation, and will NOT release the lab test reports they admit they have.
Could be HUGE mess:
@GovCanHealth
cbc.ca/news/canada/mo…



My BIGGEST concern with these BYD masks is 4-hour exposure limit.
They lied to me when I asked for an explanation, and will NOT release the lab test reports they admit they have.
Could be HUGE mess:
@GovCanHealth
cbc.ca/news/canada/mo…




Today I made this video of the BYD package.
IMHO, @BYDCompany is being deceptive about the 4-hour Exposure Limit:
• on its website, BYD discloses it under “WARNING ⚠️ Limitations of Use”
• on its package, BYD hides it on the opposite end of the box, under “Materials”.
IMHO, @BYDCompany is being deceptive about the 4-hour Exposure Limit:
• on its website, BYD discloses it under “WARNING ⚠️ Limitations of Use”
• on its package, BYD hides it on the opposite end of the box, under “Materials”.
I'm an engineer w decades in scientific & business due diligence, procurement incl Chinese contract manufacturers, and years of experience with how Kenney does things.
And this is hard for me to crack from the outside.
How does any parent know THIS is non-medical AND unsafe?!



And this is hard for me to crack from the outside.
How does any parent know THIS is non-medical AND unsafe?!




@jvipondmd @TehseenLadha @DrPeterNieman @GosiaGasperoPhD @BarryHunt008 @LeylaDAsadi @CAPPEM2 @KevinHedges15 @JenniferKShea @Lauren_Global Angry @jkenney. He & @AdrianaLaGrange don't have BSc between them, yet are Infectious Disease & Child Psych experts.
Is he mad at teachers for wanting to stick to @CMOH_Alberta Safe School plan?
Or is he mad he can't avoid big inventory writedowns now?
Is he mad at teachers for wanting to stick to @CMOH_Alberta Safe School plan?
Or is he mad he can't avoid big inventory writedowns now?
https://twitter.com/jkenney/status/1491564110516940800?s=20&t=7OcBTguvszMPPq-SDFJEaw
@jvipondmd @TehseenLadha @DrPeterNieman @GosiaGasperoPhD @BarryHunt008 @LeylaDAsadi @CAPPEM2 @KevinHedges15 @JenniferKShea @Lauren_Global @jkenney @AdrianaLaGrange @CMOH_Alberta When someone with more than a BSc, @CMOH_Alberta with MD + MPH, is asked why Safe School plan to distribute 65.6m masks over 8 weeks is stopped one-fourth of the way in, she punts to @JasonCoppingAB.
Who is trained as an employer-side labour lawyer.
Who is trained as an employer-side labour lawyer.
https://twitter.com/TheBreakdownAB/status/1492173969792778243?s=20&t=7OcBTguvszMPPq-SDFJEaw
Unfortunately, Kenny & LaGrange have only tried to kill the DEMAND for all the masks they distributed.
Good ones and bad, which nobody can differentiate without the boxes.
They have not addressed their SUPPLY. What needs to be quarantined and recalled?
@GovCanHealth
Good ones and bad, which nobody can differentiate without the boxes.
They have not addressed their SUPPLY. What needs to be quarantined and recalled?
@GovCanHealth
@GovCanHealth There MUST be Recalls.
Some parents were able to buy respirators.
Many, many parents are dependent on "medical masks" from the province, which GoA has told everyone are better than cloth masks.
Other families are giving them their BYD masks. They could circulate for months.
Some parents were able to buy respirators.
Many, many parents are dependent on "medical masks" from the province, which GoA has told everyone are better than cloth masks.
Other families are giving them their BYD masks. They could circulate for months.
This story from Quebec daycares & schools gives an idea of problems caused when Gov ignores 4-hour exposure limit the manufacturer admits, and lies that the masks have been tested.
Tx @smbilodeau & Canadian Aerosol Transmission Coalition for the help.
cbc.ca/news/canada/mo…
Tx @smbilodeau & Canadian Aerosol Transmission Coalition for the help.
cbc.ca/news/canada/mo…
@smbilodeau GoA could get into big trouble for REPEATEDLY making false claims the millions of masks they've been distributing:
"medical-grade mask has been tested and meets international standards."
Pls don't back down @GovCanHealth & Fed Min of Health @jyduclos.
canadianlawyermag.com/practice-areas…
"medical-grade mask has been tested and meets international standards."
Pls don't back down @GovCanHealth & Fed Min of Health @jyduclos.
canadianlawyermag.com/practice-areas…
This thorough April 2020 @VICENews article by @dnewhauser @keegan_hamilton details concerns with @BYDCompany fast shift into N95, KN95 and Medical Mask production, and California's US$1 billion purchase.
Regulatory concerns abound. I've seen why here.
vice.com/en/article/qjd…
Regulatory concerns abound. I've seen why here.
vice.com/en/article/qjd…
This May 2020 followup by @VICENews @dnewhauser reports that NIOSH failed @BYDCompany N95 for lack of documentation.
Same reason Australia rejected BYD's Surgical Mask.
I'm facing BYD's refusal to disclose their lab reports for the FE2411 Kids Masks.
vice.com/en/article/bv8…
Same reason Australia rejected BYD's Surgical Mask.
I'm facing BYD's refusal to disclose their lab reports for the FE2411 Kids Masks.
vice.com/en/article/bv8…
In a series of stories, independent journalists @CalMatters @LaurelRosenhall @DanielMorain have been digging into California's procurement of $1b of respirators and masks from @BYDCompany.
I've been doing the same thing in Alberta, Canada.
calmatters.org/health/coronav…
I've been doing the same thing in Alberta, Canada.
calmatters.org/health/coronav…
So if (American) investigative journalists like @CalMatters @LaurelRosenhall @DanielMorain @VICENews @dnewhauser @keegan_hamilton want to dig into @BYDCompany behaviour and government shenanigans in Canada, pls read the long thread above.
DMs open.
DMs open.
Dear Education Critic @shoffmanAB
Please ask Education Min for all lab test reports & analyses for all masks distributed in this program, starting with the BYD Children's Masks.
"A medical-grade mask has been tested and meets international standards."
alberta.ca/release.cfm?xI…
Please ask Education Min for all lab test reports & analyses for all masks distributed in this program, starting with the BYD Children's Masks.
"A medical-grade mask has been tested and meets international standards."
alberta.ca/release.cfm?xI…
• • •
Missing some Tweet in this thread? You can try to
force a refresh