
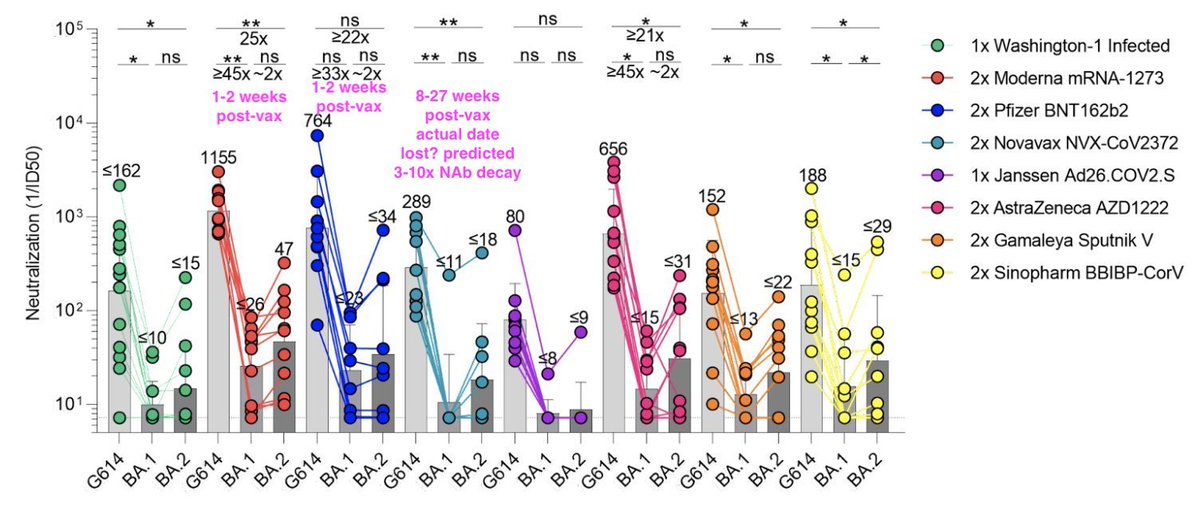
Nice study but the Novavax samples taken 55-191 days after vaccination, whereas RNA samples were taken 14 days at peak. You'd expect a 3- to 10-fold decay in that time. If you adjust for that, then Novavax looks similar on original and better on Omicron than RNA vax.
https://twitter.com/veeslerlab/status/1504274269156511745
The imbalance in collection times is seen in this table. It also seems to show a recordkeeping problem with the Novavax samples; for each sample two possible collection times. It seems they don't know which is correct? That should disqualify the data actually. 

I didn't want to conclude anything about Novavax from this paper as a result, but I was asked about it and I see it's being presented on twitter. So now I'm explaining why I think any comparison between Novavax and other vaccines is questionable.
Meaning there clearly needs to be some adjustment of the Novavax numbers. The best-guess adjustment of 3x-10x would make Novavax Abs consistent with other studies such as my favorite one here looking at peak Ab levels across vaccines.
nature.com/articles/s4159…
nature.com/articles/s4159…
And the Novavax Omicron NAb levels are so low after the decay that the mean measurement is unreliable, but if you took that unreliable number and multiply by an unreliable 3-10x, it would indeed predict better Omicron immunity than RNA vax. But did I mention that's unreliable?
So without knowing which collection time is correct, and with waned immunity reducing measurement reliability, the Novavax data are just not interpretable; better to skip it and wait for better samples. (In fact I've never seen a paper use samples with unknown collection times.)
And here's the data from Novavax's 12/2021 study showing the extent of NAb decay: >10x over 168 days from last dose (which is at 21 days)
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…
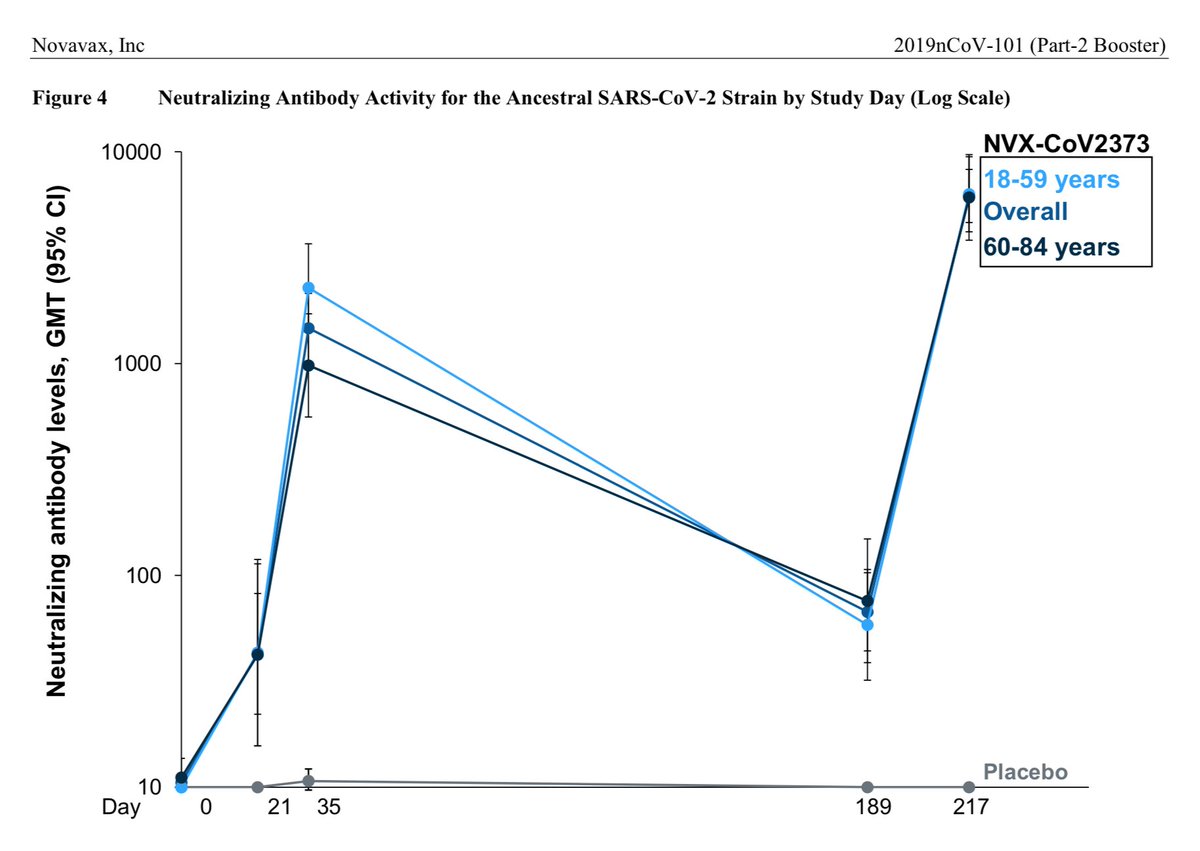
I wasn't going to show the chart from the current study given the uninterpretable Novavax results, but guess I should so we can see what is being discussed. So here's the chart with the caveats spelled out. 
• • •
Missing some Tweet in this thread? You can try to
force a refresh










