Let's talk about the Data and Safety Monitoring Committee of the TOGETHER trial.
And if committees sound boring, I promise this 🧵 will be anything but.
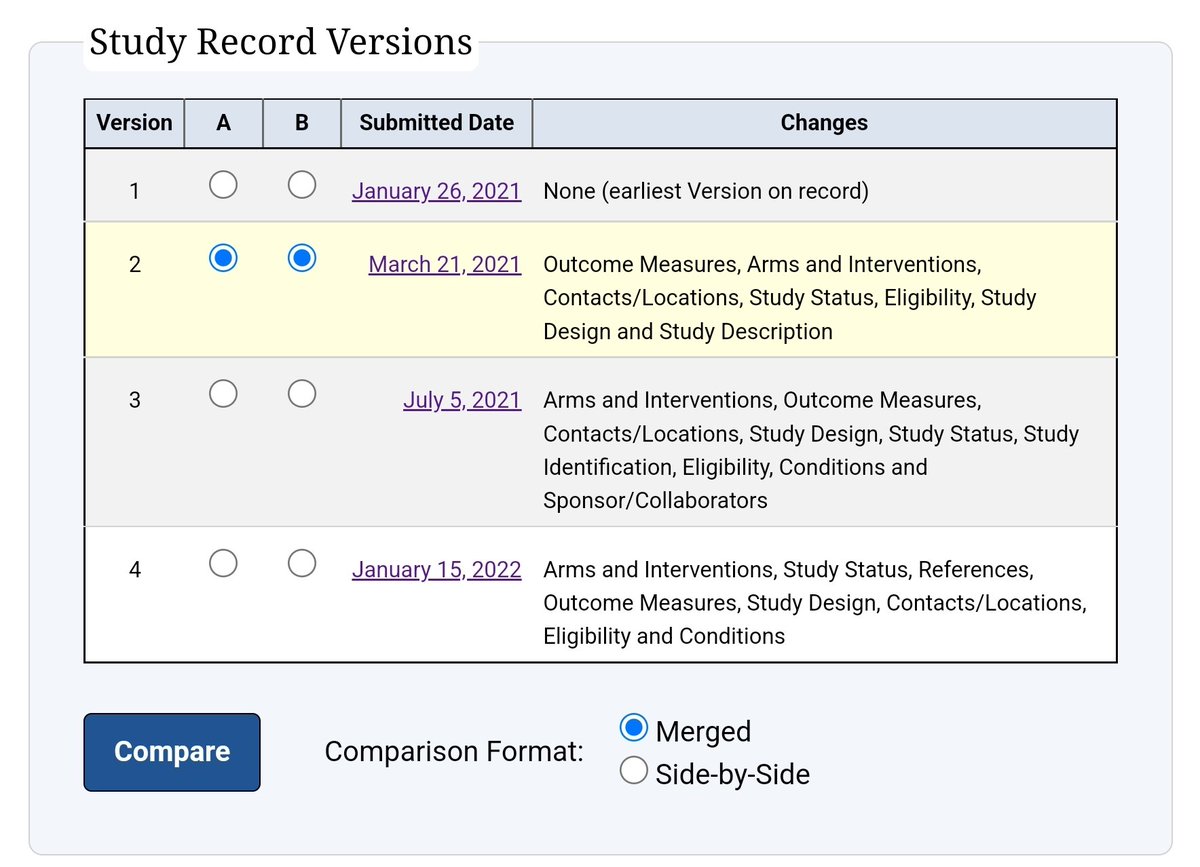
Every version of the TOGETHER trial protocol available contains this paragraph:
And if committees sound boring, I promise this 🧵 will be anything but.
Every version of the TOGETHER trial protocol available contains this paragraph:

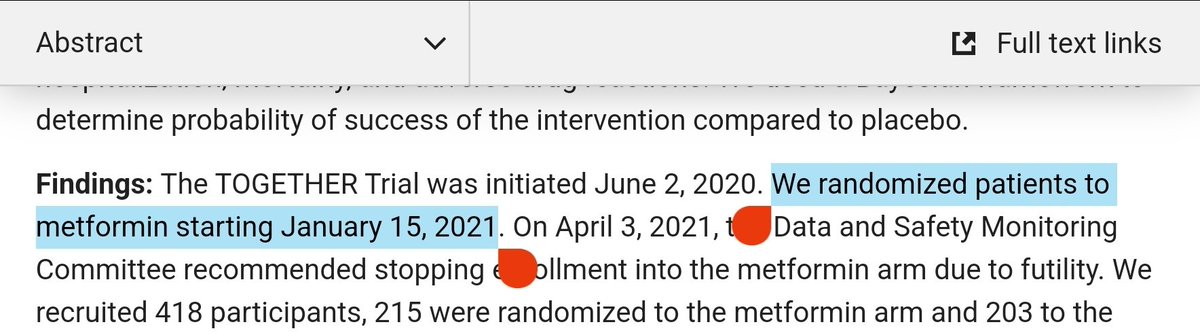
So who is in the DSMC for the TOGETHER trial? The ivm paper supplemental appendix gives us some names: 

So, doing a basic check, Orbinski seems to have co-written 9 publications with Edward Mills, the mastermind behind the TOGETHER trial. That sounds significant, but perhaps it's OK. 

Sonal Singh has a more substantial co-authorship connection with Mills, having written 29 papers together. 
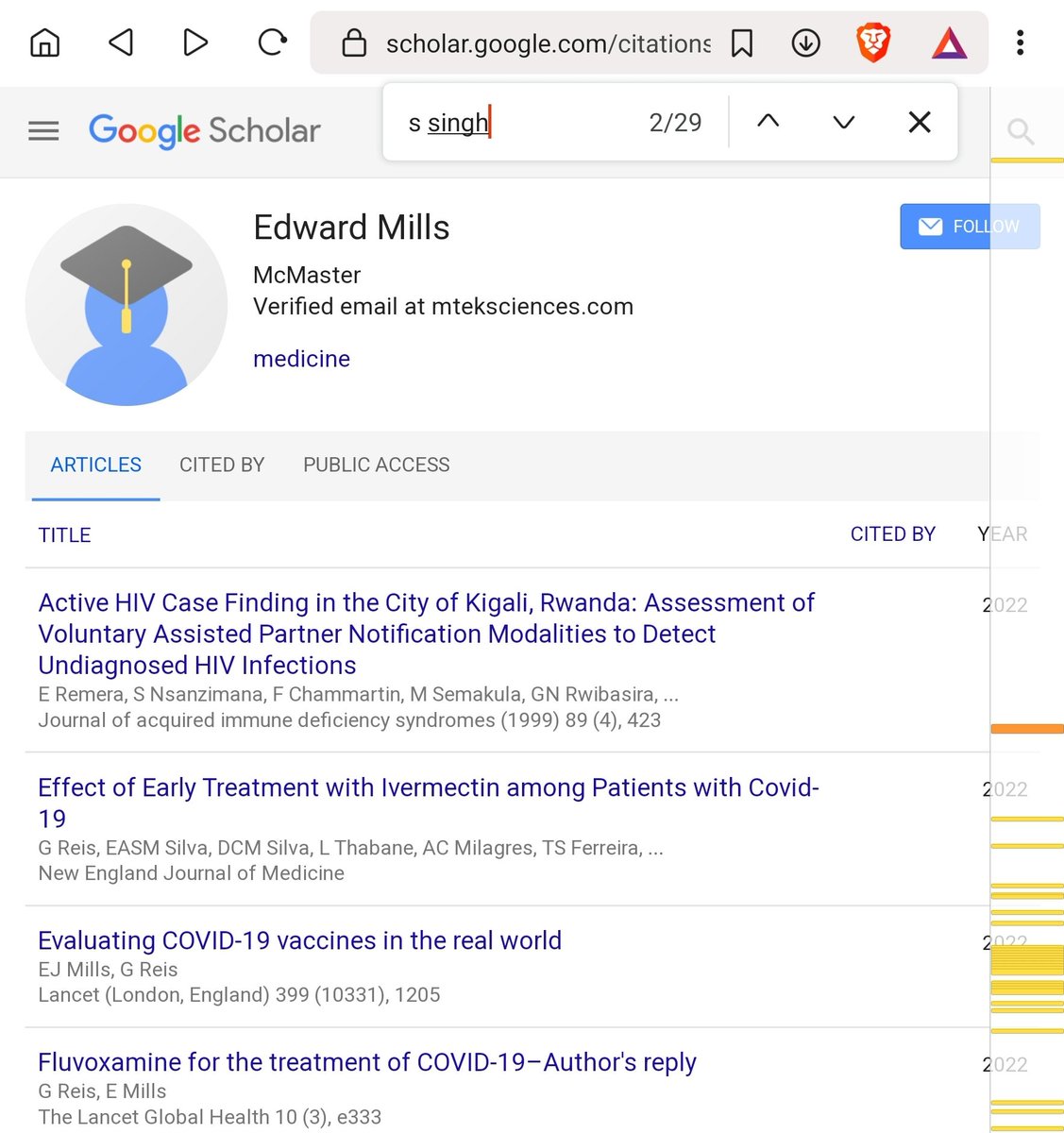
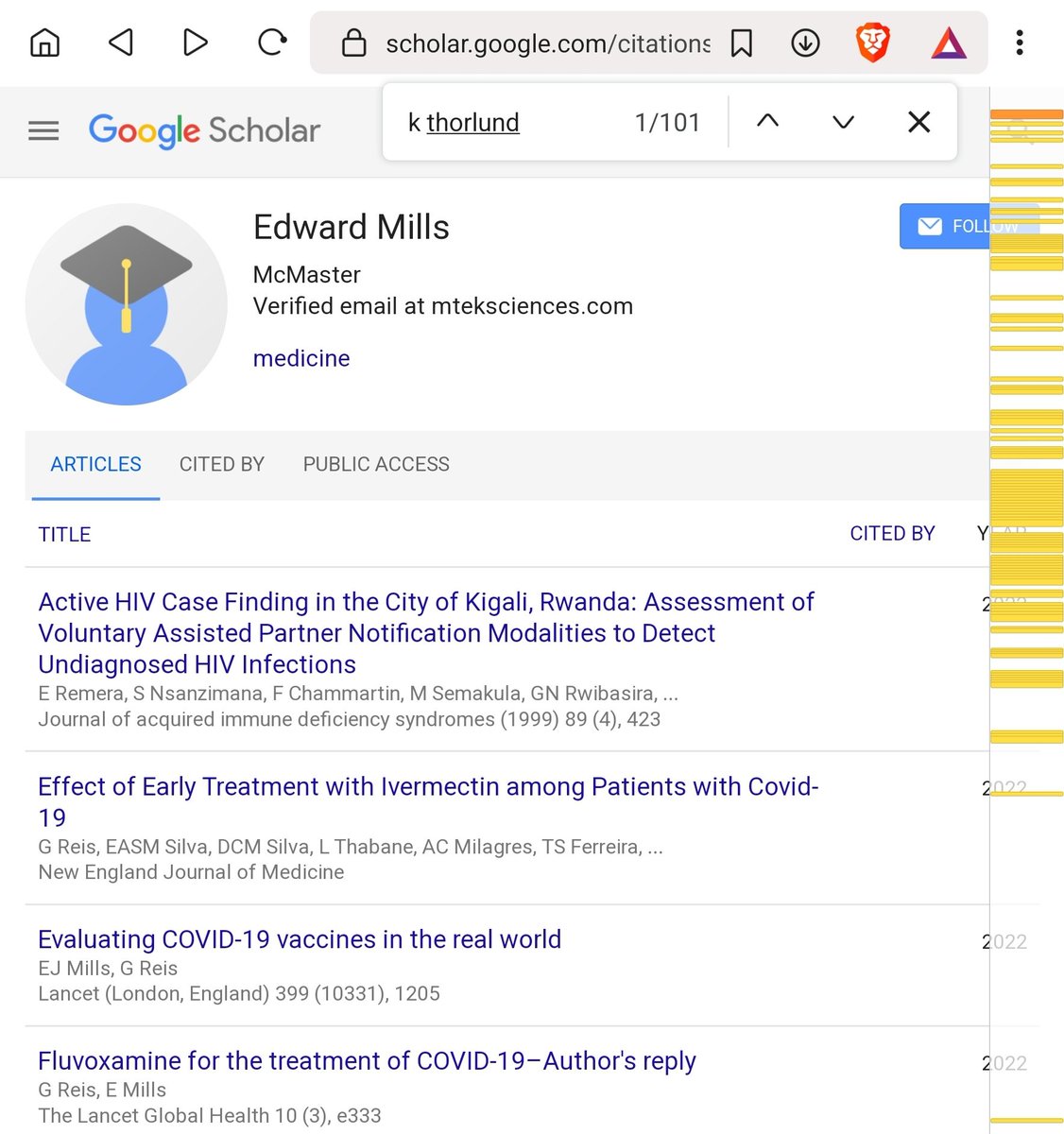
What about Thorlund? Wait, what?
... They've literally written over 100 papers together. They really seem to be tight.
... They've literally written over 100 papers together. They really seem to be tight.
The wayback machine gives us the clue we need. When the trial started, in the first version of the website, this FAQ refers to Mills and Thorlund as joint leads of the project. Not quite so independent then? 

There's something else that's strange about the first version of that page though. It points all emails to a company I haven't heard before, called MTEK Sciences. I wonder who they are. 

Searching for that name shows a lot of very interesting material. This page here has quite the trove, including 2 grants from the BMGF. devex.com/organizations/…
However it is this paragraph that is most familiar:
However it is this paragraph that is most familiar:

Looking into the concept of the Highly Efficient Clinical Trial, this paper shows up: researchgate.net/publication/33… 

A few things of interest: MTEK employed both Thorlund and Mills. One more relevant name: Jonas Haggstrom. He is also part of the DSMC of TOGETHER.
Finally, both Mills and Haggstrom were also simultaneously affiliated with the BMGF, who funds the TOGETHER trial.
Finally, both Mills and Haggstrom were also simultaneously affiliated with the BMGF, who funds the TOGETHER trial.
Trying to understand what happened to MTEK I hit the jackpot: Acquired by CYTEL in 2019, and Mills as well as Thorlund are referred to as "Founding Partners and Directors". It's basically their startup.
I can't help but wonder if MTEK stands for "Mills Thorlund Edward Kristian".

I can't help but wonder if MTEK stands for "Mills Thorlund Edward Kristian".


Given all that, I'm not sure how this DSMC can be considered independent. Thorlund seems just as invested in the success of the research protocol as Mills, and Haggstrom seems to also be very tightly linked (and still employed by CYTEL). Surely someone noticed? 

Actually someone did. In the open peer review of the protocol, this very issue was raised by a pair of Danish reviewers: 



The reviewers did not budge and did not give the protocol their full approval, since Mills refused to remove Thorlund as chair of the DSMC and only took his vote away.
Why would they insist on having Thorlund on an "independent" Data and Safety Monitoring Board?
Why would they insist on having Thorlund on an "independent" Data and Safety Monitoring Board?

The authors give their final response with two references. One is the trial design paper with Mills and Thorlund as co-authors, and the other is literally a book on Data Monitoring Committees in Clinical Trials. This might be Danish humor. 



It is stunning that the authors not only appointed a clearly non-independent DSMC for their trial, they actually more or less ignored direct reviewer advice pointing this out. And yet they insist repeating on every version of the protocol that the DSMC will have no involvement... 

• • •
Missing some Tweet in this thread? You can try to
force a refresh