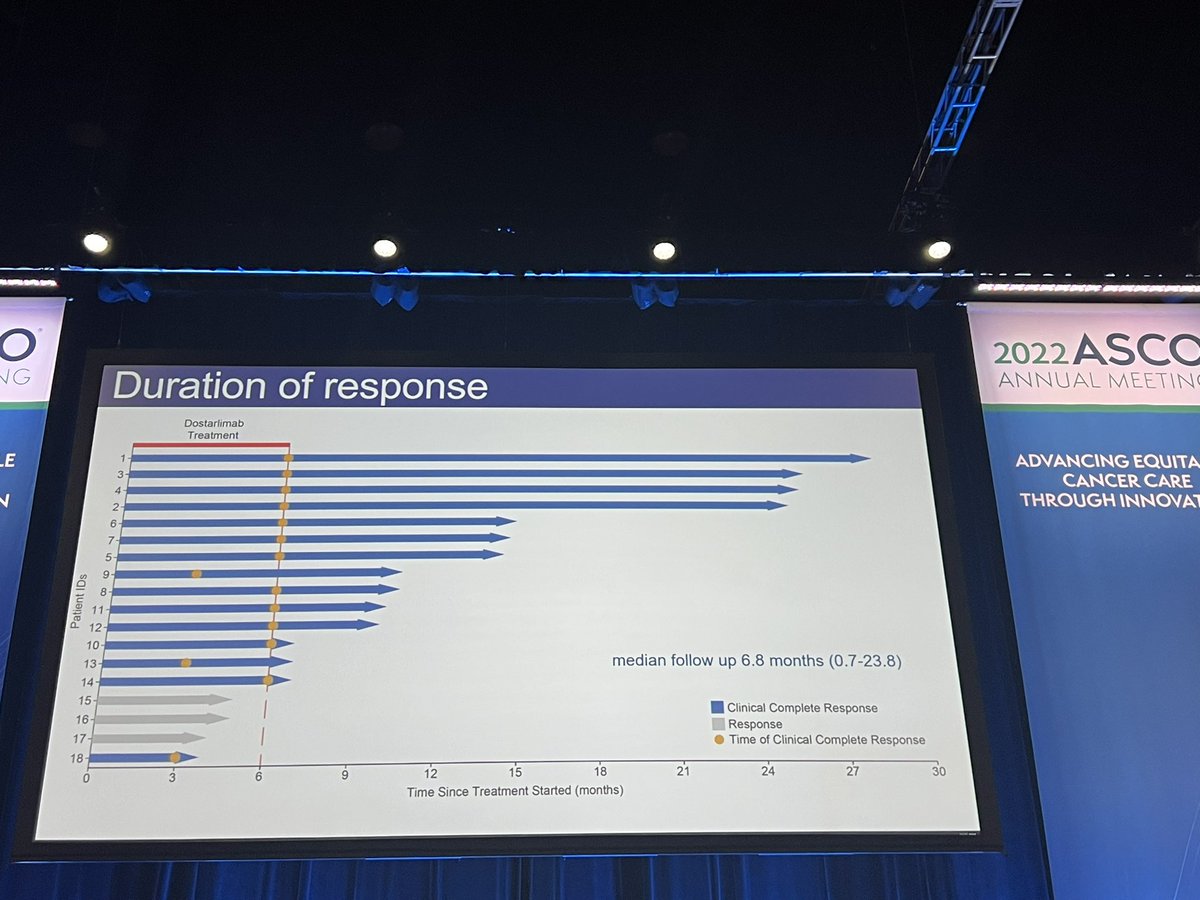
#ASCO22
✳️DYNAMIC trial results are highly exciting and glad to see field is moving forward !
✳️Very encouraging findings that we are in right pathway to use #ctDNA for adjuvant tx‼️
However there are important points that we need to address 👇@ASCO @OncoAlert @MoffittNews



✳️DYNAMIC trial results are highly exciting and glad to see field is moving forward !
✳️Very encouraging findings that we are in right pathway to use #ctDNA for adjuvant tx‼️
However there are important points that we need to address 👇@ASCO @OncoAlert @MoffittNews


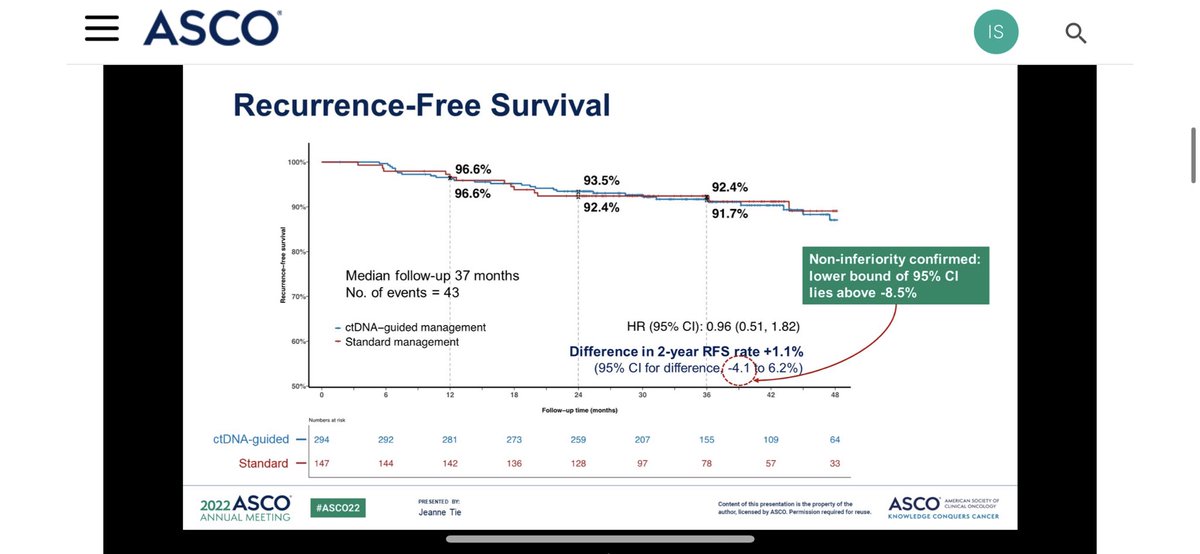
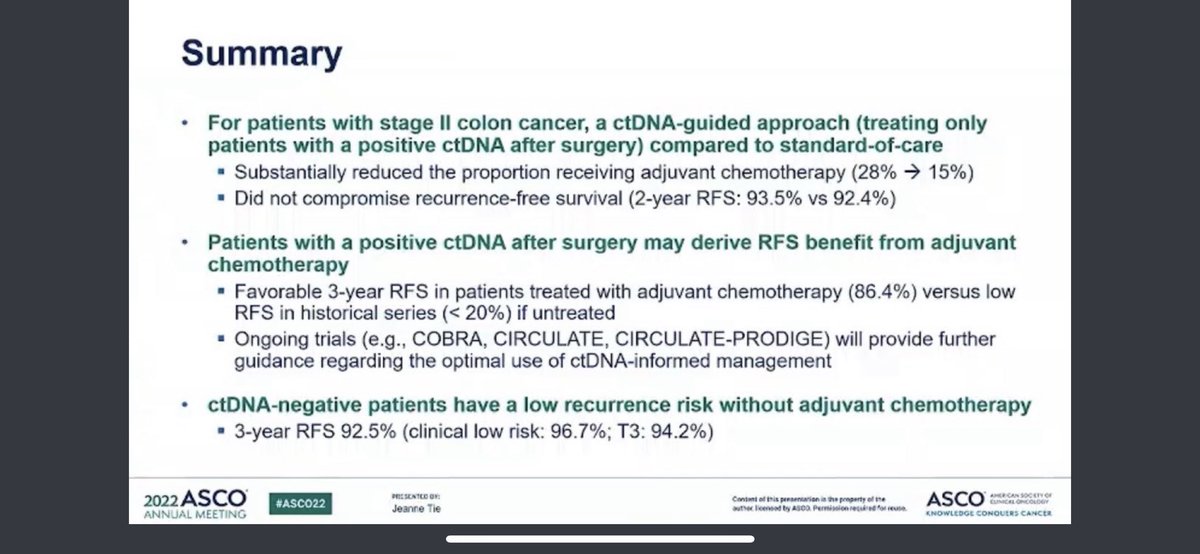
✳️Firstly, I am categorically against putting T4 and T3 disease into same pocket (stage II is highly heterogeneous)❗️
✳️Risk of peritoneal recurrence for T4 and T3 is not same and not even close ‼️
✳️Peritoneal microscopic disease doesn’t shed CtDNA as much as liver MRD‼️
✳️Risk of peritoneal recurrence for T4 and T3 is not same and not even close ‼️
✳️Peritoneal microscopic disease doesn’t shed CtDNA as much as liver MRD‼️
✳️Moreover, control group was a bit vague in this study, conventional risk factors are used for ad therapy which can offer you different approaches ‼️
✳️Currently there is not consensus for high risk stage II in NCCN and options include observation vs FP alone or doublet ‼️
✳️Currently there is not consensus for high risk stage II in NCCN and options include observation vs FP alone or doublet ‼️
✳️So this perhaps brought quite significant heterogeneity in control group and this was perhaps the reason why CtDNA guided arm received more oxaliplatin based therapies than ‼️
✳️While we are trying to go beyond clinicopathological classification, using the same system to define stage II colon population enrolled in DYNAMIC trial is my concern ‼️
Nonetheless, I personally think this study deserved to be plenary abstract for CRC❗️
• • •
Missing some Tweet in this thread? You can try to
force a refresh