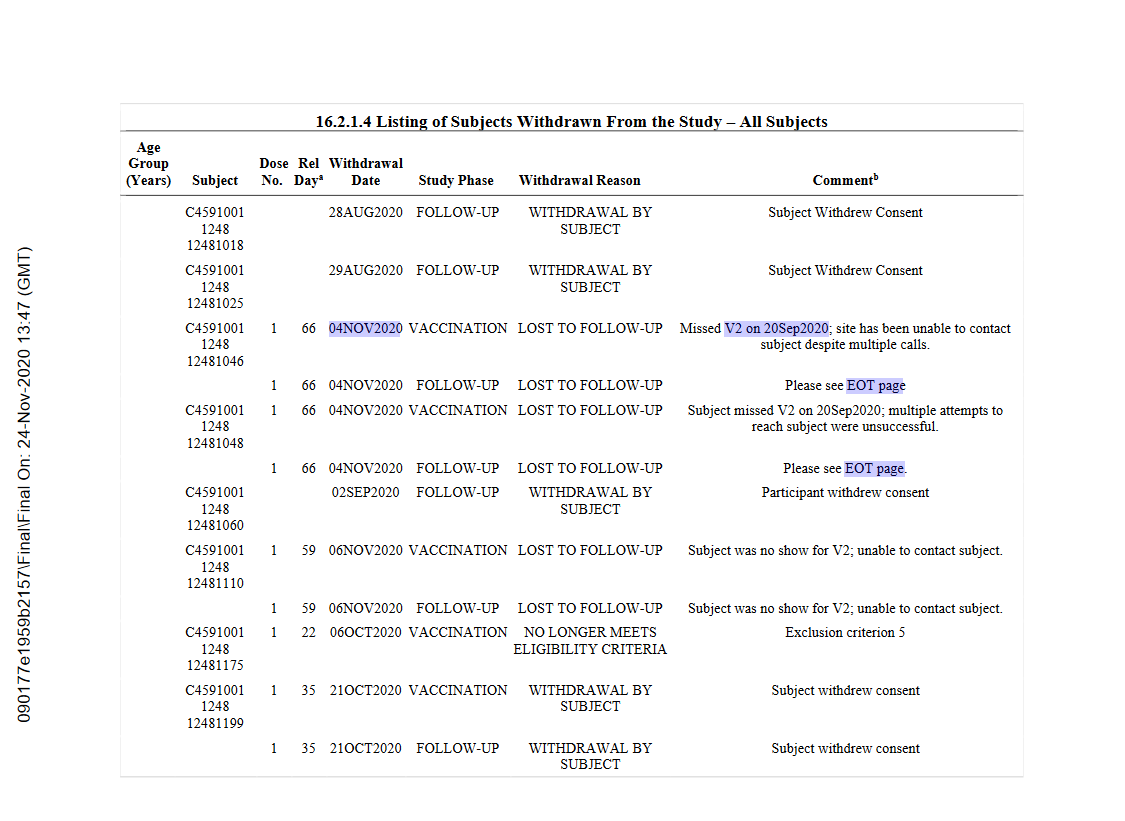
" Listing of Subjects Withdrawn From the Study"
phmpt.org/wp-content/upl…
first two pages tell us that no patients of the phase 1 trial discontinued between doses. however, the 100ug data was not used in the calculations, despite the participants being boostered with a 10ug dose..
phmpt.org/wp-content/upl…
first two pages tell us that no patients of the phase 1 trial discontinued between doses. however, the 100ug data was not used in the calculations, despite the participants being boostered with a 10ug dose..

.., because the original 100ug dose had a shit safety profile! as for the first table, could it be referring to the bnt162b1 cohort, whose data also wasn't used? 

so phase 2, the 180/180 group, only had one withdrawal? it appears so, see linked tweet#1
however, how did they manage so much better dose1+dose2 compliance compared to bnt162-01 b2 group and phase 3, as we're about to see?
https://twitter.com/a_nineties/status/1535996348469755905?s=20&t=oyvquNWlFgp4SWBNGVhwuw
however, how did they manage so much better dose1+dose2 compliance compared to bnt162-01 b2 group and phase 3, as we're about to see?

here we go. all listed withdrawals phase 2/3 and open-label unblinded period, p 4/112! i'm scraping this manually to an excel file, see how long it takes 

this poor person was discontinued for testing positive 21 days after their 2nd dose. wait what?? shouldnt that go into efficacy calcs?? 

an hiv+ pregnancy 🤨😳😬the cross at the end of the pt# signifies hiv infection. 22 days after the jab... wonder how the baby's doing. and a screenshot of my excel autism 



ALERT ALERT new disease CODIV-19 reported in argentina! #site1231
PAGING DR POLLACK, I REPEAT, PAGING DR POLLACK
YOUR PRESENCE IS REQUIRED IN THE HUMAN RESOURCES DEPARTMENT
PAGING DR POLLACK, I REPEAT, PAGING DR POLLACK
YOUR PRESENCE IS REQUIRED IN THE HUMAN RESOURCES DEPARTMENT

you come up with that _after_ your jab? poor kid! also, #site1231 has the vast majority of pos+covid withdrawals 🤨🤨🤨🤨🤨🤨🤨🤨🤨 

was it perhaps... exclusion criterium #5, aka codiv-19? we may never know. wonderhow many more CRFs are in the pipeline 

patient 1149 of site 1247, hope you're still alive, because i'm having some trouble believing someone would move without back notice one day after enrolling in a study. 

a clinical diagnosis of covid-19 ONE day after first dose. if they're specifying clinical, does that mean they didnt have a positive pcr? would love to see that CRF 

refusing a swab after dozens of swabs, jabs, blood draws? #site4444 pt1224 would have been in the evaluable efficacy population. did 1224 refuse, or were they refused? 

the only two withdrawals due to "travelling abroad" (in a pandemic) are two consecutive withdrawals from #site4444. for the coincidence theorists among us, thats a tough one to swallow. 

done with the 18-55 age group. there were 117 covid-positive withdrawals across all sites.
53 of those occurred at #site1231, which we know enrolled 10% (4501 pts) of the total study pop.
what the hell are they gonna release next month?


53 of those occurred at #site1231, which we know enrolled 10% (4501 pts) of the total study pop.
what the hell are they gonna release next month?
https://twitter.com/Jikkyleaks/status/1523617240062791680?s=20&t=-XuNjk6OtWOzmQL-KG-ljw


"mr PI, why isn't patient 1141 returning his calls? should we call the emergency contact?"
"no!, no need, i talked to him, he's just had to move out of country, you know how it is..."
"no!, no need, i talked to him, he's just had to move out of country, you know how it is..."
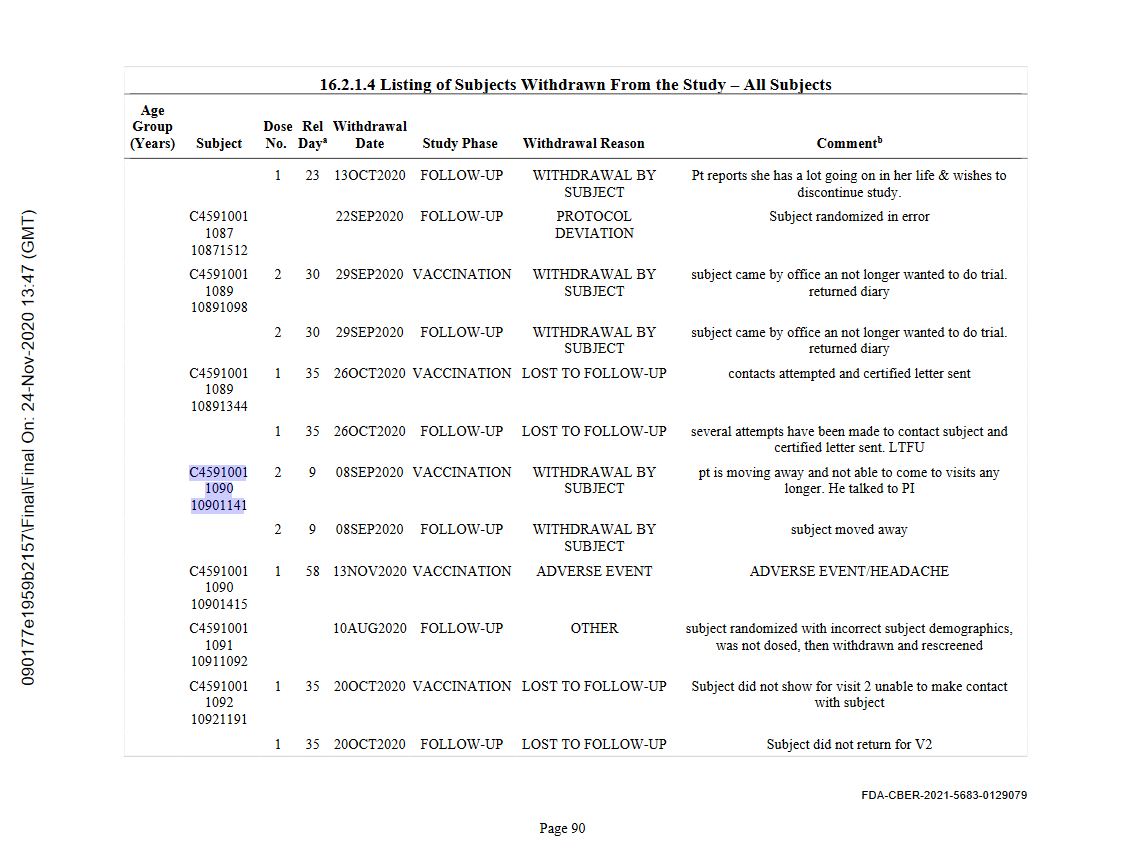
"withdrawal by subject due to ongoing medical issues" 33days after 2nd jab, yeah i'm gonna list that as an AE 

• • •
Missing some Tweet in this thread? You can try to
force a refresh