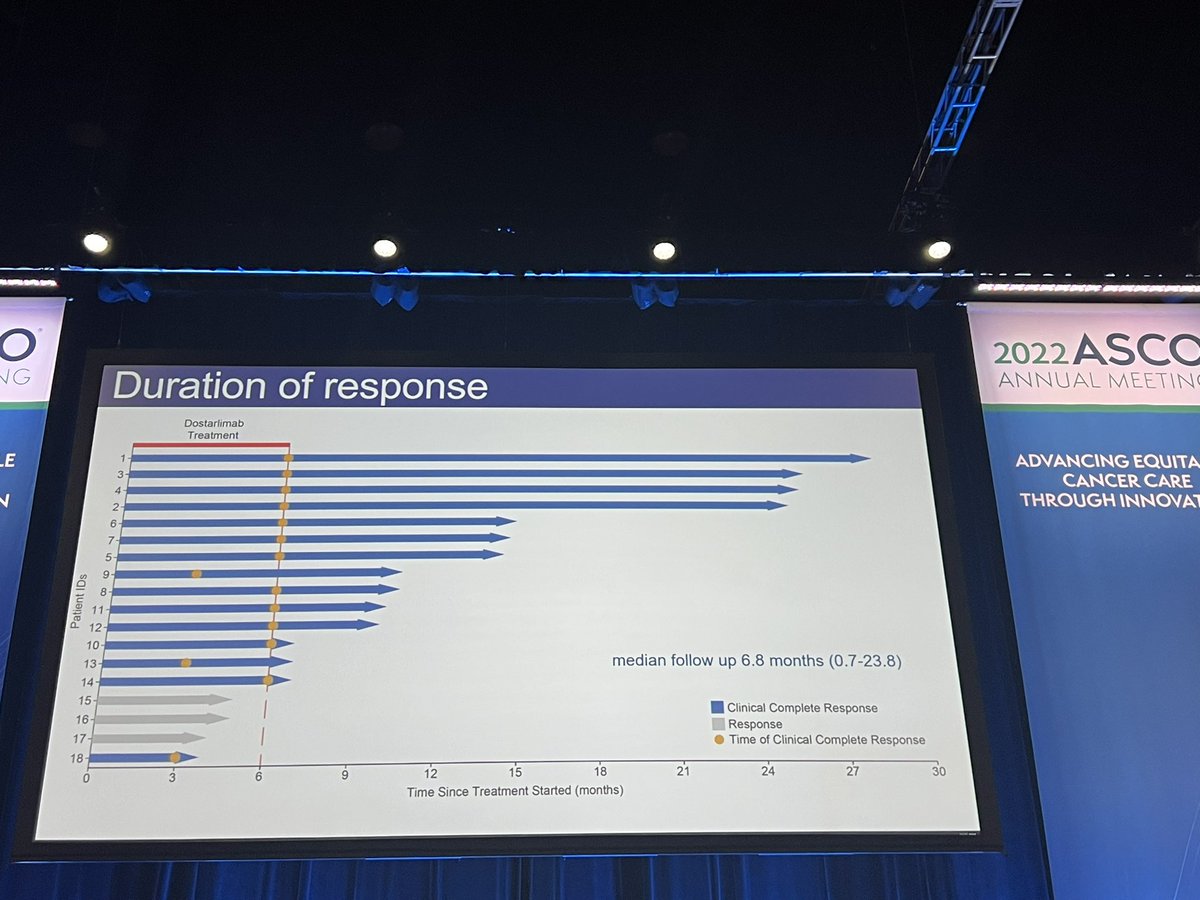
I would like to do my 2nd deep dive post #ASCO22 analysis on #ctDNA guided adjuvant therapy for pts with stage II colon cancer‼️
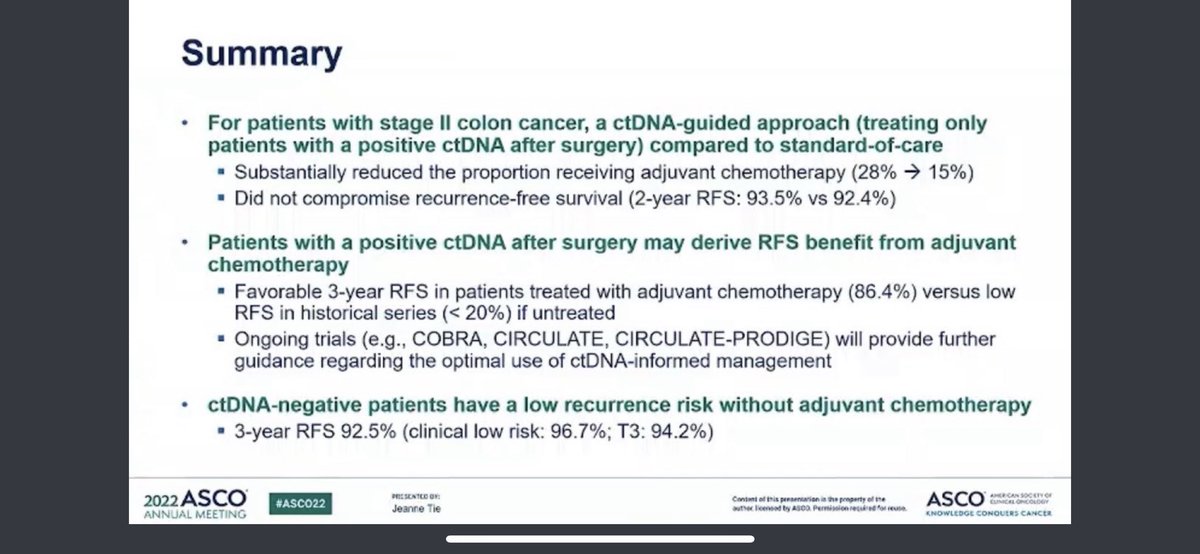
✳️The DYNAMIC trial is certainly step forward (kudos) ‼️But doesn’t answer many questions❗️@OncoAlert @manjuggm @CrcChange @TheColonClub Why ?👇
✳️The DYNAMIC trial is certainly step forward (kudos) ‼️But doesn’t answer many questions❗️@OncoAlert @manjuggm @CrcChange @TheColonClub Why ?👇

1️⃣ Firstly, the whole point of ctDNA is identification of MRD so to give RIGHT chemotherapy to RIGHT patients❗️
✳️Not only reduce adjuvant tx, but offer it to patients who may potentially miss that opportunity (such as offering folfox to a patient with ctDNA (+)T3N0 disease)❗️
✳️Not only reduce adjuvant tx, but offer it to patients who may potentially miss that opportunity (such as offering folfox to a patient with ctDNA (+)T3N0 disease)❗️
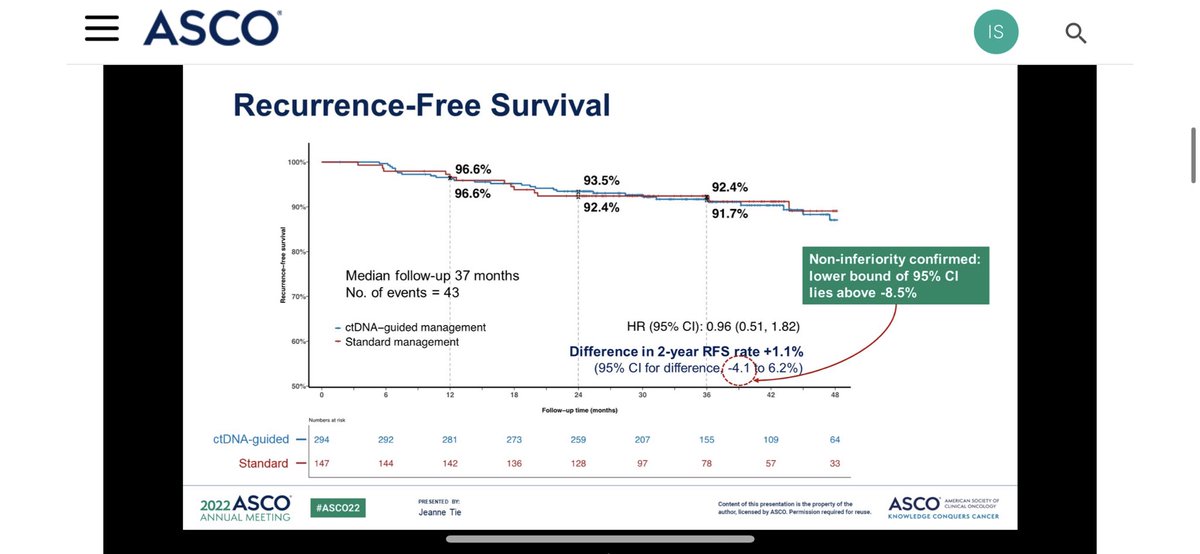
2️⃣The DYNAMIC suggests we get same DFS rate whether we use ctDNA or conventional risks for decision‼️
✳️Then one would rightfully wonder why to use ctDNA over conventional clinicopathological criteria if we get same outcomes anyway by using MORE oxaliplation❓just to reduce 5FU❓
✳️Then one would rightfully wonder why to use ctDNA over conventional clinicopathological criteria if we get same outcomes anyway by using MORE oxaliplation❓just to reduce 5FU❓
3️⃣One would also criticize that similar outcomes are driven by the impact of T4 disease included in this study (potential false negatives receiving no therapy in ctDNA directed arm)‼️
✳️While the same patient could have received adj therapy in standard arm‼️
✳️While the same patient could have received adj therapy in standard arm‼️
3️⃣ I think, just reducing chemotherapy should not be endpoint of ctDNA trials moving forward as we try to deliver ajd tx more precisely to avoid both OVER and UNDER treatment❗️
✳️T3N0 only with superiority design by use of FOLFOX would be more exciting and convincing for me‼️
✳️T3N0 only with superiority design by use of FOLFOX would be more exciting and convincing for me‼️
4️⃣We may also need to avoid using control arms with historical approach as inherently this may bring heterogeneity to studies‼️
✳️All patients should undergo CtDNA testing before enrolled and should included in control arm.
✳️All patients should undergo CtDNA testing before enrolled and should included in control arm.
5️⃣ A nice example of ctDNA based trial design is CIRCULATE-US ❗️ Why ? As approaches tries to de-escalate in low risk group (ctDNA (-) non inferiority) and escalate in ctDNA + arm (superiority)‼️
✳️Therefore we need to enroll in ongoing US trial to learn more about ctDNA‼️
✳️Therefore we need to enroll in ongoing US trial to learn more about ctDNA‼️
6️⃣Overall, the DYNAMIC trial is step-forward for reliability of ctDNA yet we have so many questions unanswered and can be answered by ongoing prospective trials ‼️
✳️Consider Enrolling CIRCULATE-US and Cobra❗️
✳️Consider Enrolling CIRCULATE-US and Cobra❗️
• • •
Missing some Tweet in this thread? You can try to
force a refresh