Live blog of #VRBPAC meeting by @HelenBranswell and @matthewherper
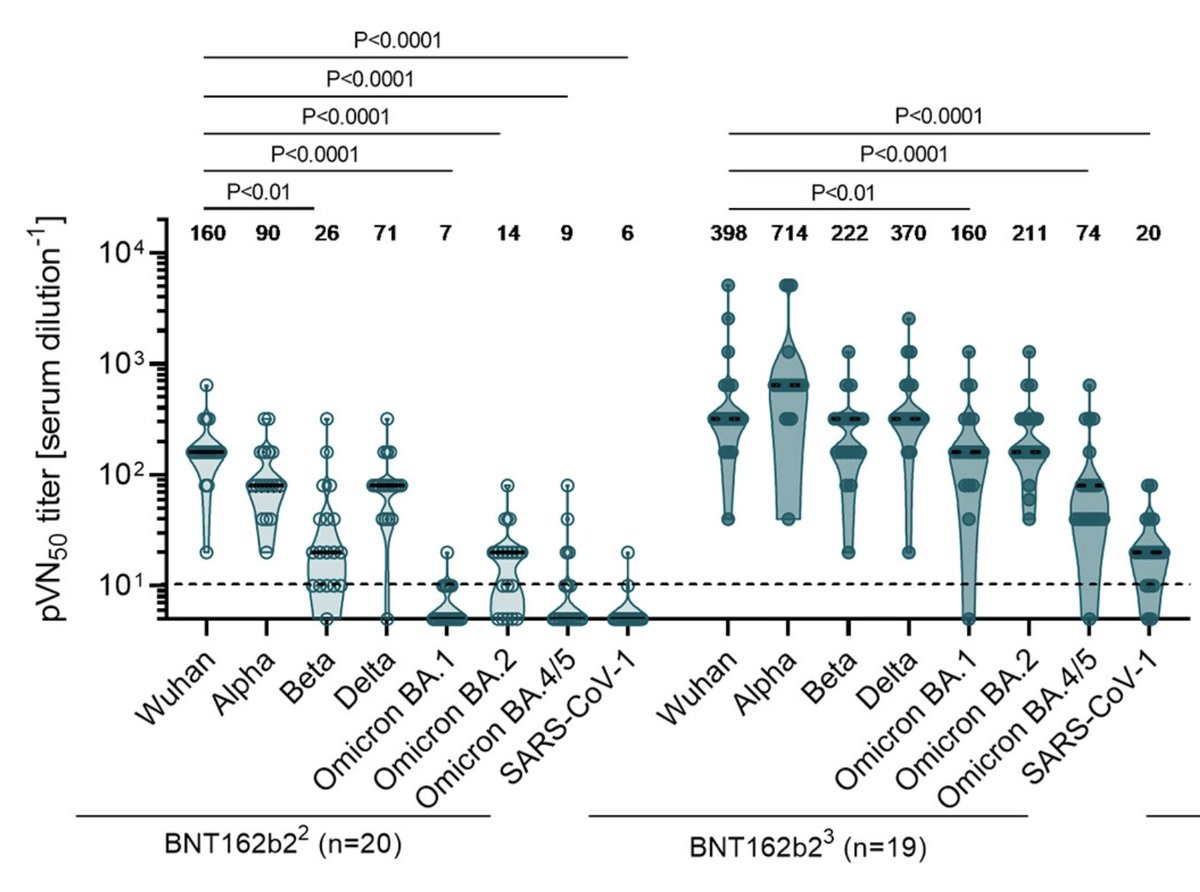
First interesting info is that VE for BA2 hospitalization ("what we should care about" since we gave up on stopping disease) is at best 71% after 3 doses. Far worse than the 95% that we had to original strain
First interesting info is that VE for BA2 hospitalization ("what we should care about" since we gave up on stopping disease) is at best 71% after 3 doses. Far worse than the 95% that we had to original strain

@HelenBranswell @matthewherper Live blog link, thx to Helen and Matthew for condensing the important points: statnews.com/2022/06/28/tra…
And in the biggest shocker of any VRBPAC I've seen, Novavax showed up. We're used to them not being invited to the party.
And not only did they show up, they actually presented new data on Omicron including BA1 BA2 and BA5.
And it was very impressive data too
h/t @doctorvasan
And not only did they show up, they actually presented new data on Omicron including BA1 BA2 and BA5.
And it was very impressive data too
h/t @doctorvasan

@doctorvasan What's even more interesting is that Novavax was willing to *say* that their vaccine seems to offer broader protection in front of the FDA. In the past they seemed wary of making this claim lest they get objections from Pfizer or FDA, two entities you don't want to upset 

I had noticed that Novavax's drop in antibody titers (either receptor inhibition or pseudovirus neutralization) going from original strain to Omicron BA1 looked less severe than with RNA in the 12/2021 study. But for a long time that was the only study.
https://twitter.com/michaelzlin/status/1488957682874679298
If you don't reproduce a finding quickly, people start to doubt whether it was a fluke. It seems with today's data that the finding of Novavax working better on variants holds up, and extends to BA4/5 too.
Why Novavax can gives broader responses is unclear but I proposed better presentation of the more conserved S2 domain of the spike protein, because Novavax abolished the S1-S2 cleavage site.
https://twitter.com/michaelzlin/status/1503491717399400450
The proteins encoded by RNA vax preferentially present S1. Even now, most studies treat this as a feature, as most neutralization activity is against S1 (including RBD). But because variants easily evolve out those epitopes, this may have become a weakness
https://twitter.com/michaelzlin/status/1503529839751430145
Predictably, Dr. Offit doubts the need for any update at all. Of course just talks about severe disease. No consideration for long COVID, morbidity, people becoming variant incubators, or lost productivity.
You can replace him with a tape recorder and have the same effect.
You can replace him with a tape recorder and have the same effect.

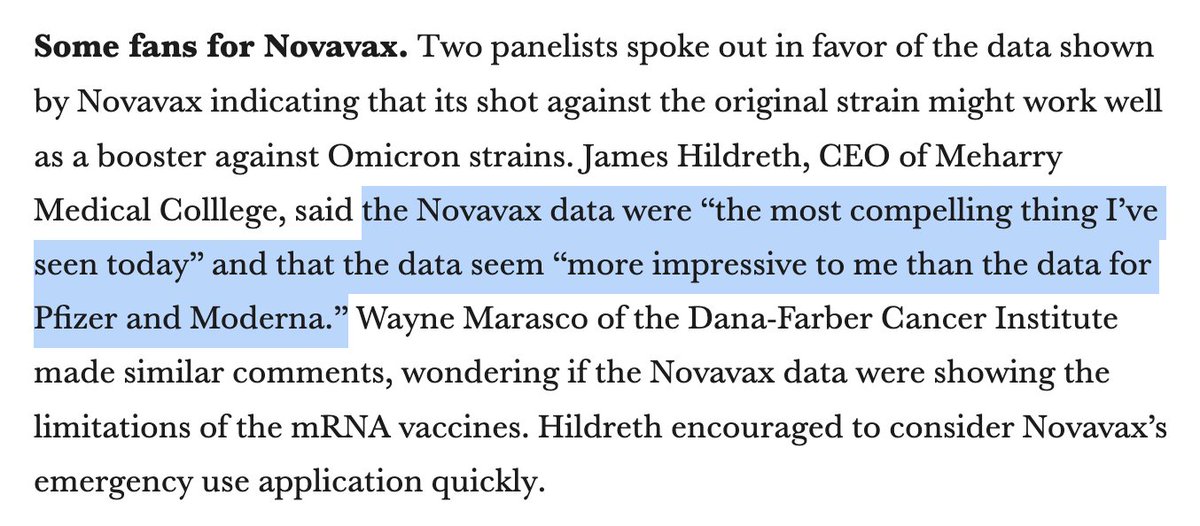
And 👏🙏 @JamesEKHildreth who actually is thinking for himself: using data to come up with a fair recommendation, not just taking cuese from government or company officials. Did the same on AMDAC. His integrity and ability are unusual. We need him in a true decision-making role. 
Oh please, not this again. Are we never going to improve our vaccines because we don't want to admit old ones are outdated?
Should we stop improving cars because it will make people who can only afford old cars feel bad?
Should we stop improving cars because it will make people who can only afford old cars feel bad?

BTW, in case you haven't noticed, VRBPAC meetings are just like twitter discussions. Same arguments, some cogent and relevant, some not. Only a few weeks later.
You can probably write a program that parses twitter threads to predict what will be said at the next VRBPAC
You can probably write a program that parses twitter threads to predict what will be said at the next VRBPAC
And the vote is yes we should update our vaccines.
Completely unnecessary. WHO already said so and VRBPAC itself said so >2mo ago in early April!
FDA seems to use VRBPAC for political cover and journalist education, not for any decision-making
Completely unnecessary. WHO already said so and VRBPAC itself said so >2mo ago in early April!
FDA seems to use VRBPAC for political cover and journalist education, not for any decision-making
https://twitter.com/michaelzlin/status/1511888823059329034
Two dissenters though. We already know who one of them is. Might as well give him voting card with only one entry: No 

But seriously, the lack of info on tangible progress on the regulatory side since the April 6 meeting is disappointing. There everyone agreed there should be a regular process for updating vaccines, but nearly 3 months later it doesn't seem like we have a process.
In fact we seem to have reduced our expectations for a regular process; last time there was talk of setting up a flu-like system for WHO guidance, then VRBPAC rubber-stamping and FDA issuance of RFAs. Today we heard FDA wonders if we should be more targeted than WHO.
So it seems for another year we will have ad-hoc applications by companies followed by FDA saying yes if you're Pfizer, wait for Pfizer if you're Moderna, and wait indefinitely if you're Novavax.
Details on the timeline: August if BA1 or original-BA1 bivalent. October if FDA orders a switch to BA2 and/or BA4/5.
politico.com/news/2022/06/2…
politico.com/news/2022/06/2…

As I said earlier, I think a switch to only BA4/5 (4 and 5 are the same S protein) would be unwise because BA2 is still going around. Also many people haven't gotten either BA1 or BA2, so they would be susceptible to BA2, in addition to BA4/5.
FDA has the data on how well Pfizer and Moderna's BA1 vax do on BA2 and BA4, so they may decide with that. If FDA wants a change to BA4±2, they can guide that BA1 may not be approvable due to failure to address the medical need, whereas BA4±2 might be if done by a certain time.
This way FDA can lead on public health policy while using the procedures of the FDA, which are designed to approve or disapprove companies' applications to sell drugs based on evidence of safety and efficacy
Some background to understand the above: BA1 and BA2 appear close antigenically; immunization by one provides decent immunity to the other. BA2.12 and BA4/5 are more distinct due to mutation of some major neutralizing epitopes even if they are close in sequence to BA2
• • •
Missing some Tweet in this thread? You can try to
force a refresh