Here's a question that you might not have considered: how did the dose for #sacubitril/valsartan in #HFrEF get chosen?
Out today is a paper in @JACCJournals led by @RezaMohebiMD that addresses some questions about sac/val dose in HFrEF.
a 🧵
jacc.org/doi/epdf/10.10…
Out today is a paper in @JACCJournals led by @RezaMohebiMD that addresses some questions about sac/val dose in HFrEF.
a 🧵
jacc.org/doi/epdf/10.10…

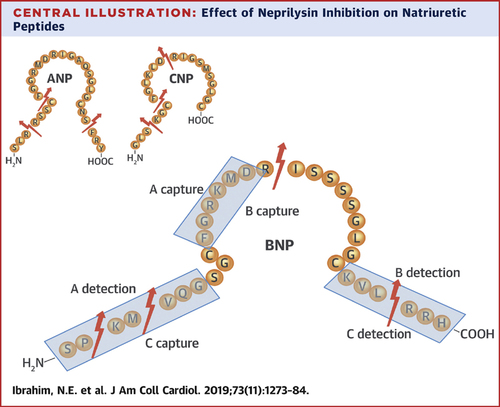
It's a little known fact that prior to the PH3 PARADIGM trial, a PH2 study in HFrEF was not performed--normally PH2 studies provide target doses for the pivotal outcomes trials.
So how was the dose of sacubitril/valsartan chosen??
So how was the dose of sacubitril/valsartan chosen??
The target dose of 97/103 mg twice daily was selected to achieve serum concentrations of valsartan = to those in Val-HeFT and VALIANT while simultaneously achieving 90% neprilysin inhibition in normal individuals.
Real world evidence suggests only 14% reach the target dose of 97/103 mg twice daily.
Some have asked--if you can't get to target dose, is sac/val still effective?
Some have asked--if you can't get to target dose, is sac/val still effective?
In the #PROVE-HF study, 794 study participants with #HFrEF (80% taking ACE/ARB at baseline) were initiated and titrated to the maximally tolerated sac/val dose.
Biomarkers and KCCQ were collected a 9 time points and an echo was performed at baseline, 6 and 12 months.
Biomarkers and KCCQ were collected a 9 time points and an echo was performed at baseline, 6 and 12 months.
We categorized patients in the basis of their average daily sac/val dose:
Total dose across all study visits
--------------------------------------
Total days in study
We then divided study participants into three groups: high dose, medium dose, and low dose.
Total dose across all study visits
--------------------------------------
Total days in study
We then divided study participants into three groups: high dose, medium dose, and low dose.

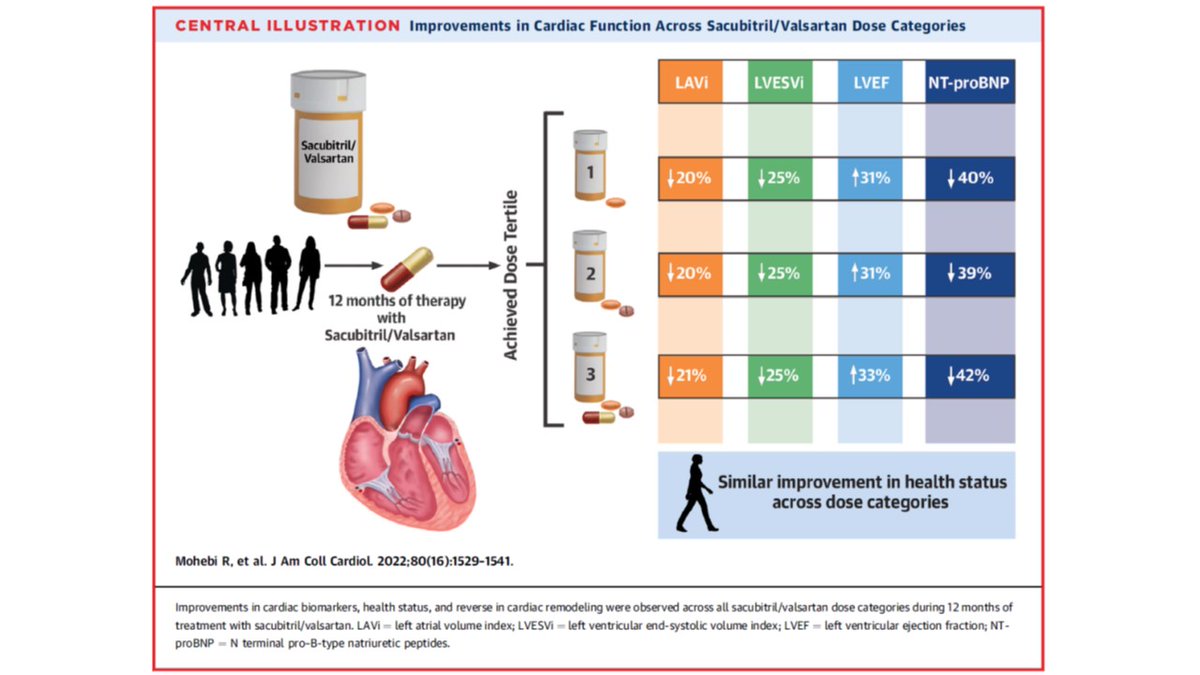
The 3 dose groups were an average of 112 mg daily, 342 mg daily, and 379 mg daily.
Those in low dose reached target least often (8%), while medium (94.5%) and high dose (100%) were more likely to reach or stay at target dose.
Baseline characteristics by achieved dose are shown.
Those in low dose reached target least often (8%), while medium (94.5%) and high dose (100%) were more likely to reach or stay at target dose.
Baseline characteristics by achieved dose are shown.

Those able to get to target on sac/val were very PARADIGM-like: younger, previously on ACE/ARB, and had higher BPs.
Those on mod dose were similar to those down-titrated in PARADIGM.
Those reaching low doses were most like those who did not make it through the PARADIGM run in.
Those on mod dose were similar to those down-titrated in PARADIGM.
Those reaching low doses were most like those who did not make it through the PARADIGM run in.
What was modestly surprising was the baseline echo data showed little differences between dosing groups. 

OK, so what about changes after study initiation?
Biomarkers? Health status? Echo?
What is your expectation? Would higher dose be associated with larger improvement in each?
Biomarkers? Health status? Echo?
What is your expectation? Would higher dose be associated with larger improvement in each?
First, biomarkers. Attached at tables for the stable (#NTproBNP; #hscTnT; #sST2; urinary #cGMP).
Regardless of dose, patterns were very similar for each: for example NT-proBNP fell by 40%, 39% and 42% in low, medium, and high dose.
Regardless of dose, patterns were very similar for each: for example NT-proBNP fell by 40%, 39% and 42% in low, medium, and high dose.

What about #ANP and #BNP?
We had previously reported that rise in ANP explained much of the reverse remodeling impact of sac/val, so one might expect a larger rise from higher doses...right?
Nope. Relative change was similar across doses.
Same for BNP.
We had previously reported that rise in ANP explained much of the reverse remodeling impact of sac/val, so one might expect a larger rise from higher doses...right?
Nope. Relative change was similar across doses.
Same for BNP.

We had reported improvement in health status (KCCQ-23) from rx with sac/val in PROVE HF: jacc.org/doi/10.1016/j.…
Was there a dose-related difference in KCCQ-23 change?
Numerically, yes. Statistically, no.
There was also no difference in a >5, 10, or 20 point rise across doses
Was there a dose-related difference in KCCQ-23 change?
Numerically, yes. Statistically, no.
There was also no difference in a >5, 10, or 20 point rise across doses

OK, so what about the echo? Higher doses must have had larger increases in LVEF, and greater reduction in LV/LA volumes and E/E' right?
Again, reductions across all measurements were strikingly similar, despite the higher risk picture in the lower dose study participants.
Again, reductions across all measurements were strikingly similar, despite the higher risk picture in the lower dose study participants.

These results do not contradict clinical practice guidelines: therapies should be titrated to maximally tolerated doses when possible.
If it is not possible to reach target, it is reassuring to note mechanistic benefits are still seen even at low dose!
#GDMTworks
If it is not possible to reach target, it is reassuring to note mechanistic benefits are still seen even at low dose!
#GDMTworks
@RezaMohebiMD @JavedButler1 @DukeHFDoc @RxPDX @scottdsolomon @gcfmd @BiykemB @MinnowWalsh @NMHheartdoc @hvanspall
@RezaMohebiMD @JavedButler1 @DukeHFDoc @RxPDX @scottdsolomon @gcfmd @BiykemB @MinnowWalsh @NMHheartdoc @hvanspall @MGH_RI @MGHHeartHealth @BaimInstitute
• • •
Missing some Tweet in this thread? You can try to
force a refresh