a brief outline of the Nov 1 PHMPT release.
mega.nz/file/yRgyQZzZ#…
these are all the BNT162-01 related .xpt files as .csv, the C4591001 file is too big to open in excel 😅 and you don't need me for the .pdf (i hope)
mega.nz/file/yRgyQZzZ#…
these are all the BNT162-01 related .xpt files as .csv, the C4591001 file is too big to open in excel 😅 and you don't need me for the .pdf (i hope)

first file is -suppex.csv. it seems to be a protocol checklist, with six rows per patient except if there was a protocol deviation. includes medication nr's. the screenshots show the only two deviations recorded in this file. 





which brings us to -suppec.csv
at a glance, it looks identical to -suppex.csv, but it isn't. while it also lists the two deviations shown above, it also lists a few more. in -suppex.csv, 1-dose patients only have three rows, -suppec.csv just provides add. info?


at a glance, it looks identical to -suppex.csv, but it isn't. while it also lists the two deviations shown above, it also lists a few more. in -suppex.csv, 1-dose patients only have three rows, -suppec.csv just provides add. info?



-suppds.csv is next. this seems to list patients by their cohort, group, and protocol version at time of enrollment.
group B is BNT162b1, group C is BNT162b2. So what's group A? will x-ref with the protocol amendments. not sure this has all Pts, will check.


group B is BNT162b1, group C is BNT162b2. So what's group A? will x-ref with the protocol amendments. not sure this has all Pts, will check.



next is suppcm.csv
this lists ATC codes (Anatomical Therapeutical Chemical) for patients that received medication (presumably due to AEs?). will be digging deeper into this one and x-ref'ing with my AE file
this lists ATC codes (Anatomical Therapeutical Chemical) for patients that received medication (presumably due to AEs?). will be digging deeper into this one and x-ref'ing with my AE file

next is -suppae.csv
this seems to be a classification file for AEs. each AE has three rows, very rarely is there a "Y" under "Dose limiting toxicity". might be interesting to compare with the actual AEs
this seems to be a classification file for AEs. each AE has three rows, very rarely is there a "Y" under "Dose limiting toxicity". might be interesting to compare with the actual AEs

next is S-D-se.csv
seems to be a timeline per patient listing intervals between screening, pre-dose assessment, vax dates, and followup.
lots of patients with "NA" under followup🤔some patients don't even have a fifth "FUP" row. will look at prev files if theres smthg to x-ref
seems to be a timeline per patient listing intervals between screening, pre-dose assessment, vax dates, and followup.
lots of patients with "NA" under followup🤔some patients don't even have a fifth "FUP" row. will look at prev files if theres smthg to x-ref

next is S-D-pe.csv
this is a list of medical assessments of different parts of the body per patient per visit(>20 rows/pt). very peculiar as many patients who had AEs get a clean sheet in this file. another one to x-ref with the AE listings.
this is a list of medical assessments of different parts of the body per patient per visit(>20 rows/pt). very peculiar as many patients who had AEs get a clean sheet in this file. another one to x-ref with the AE listings.

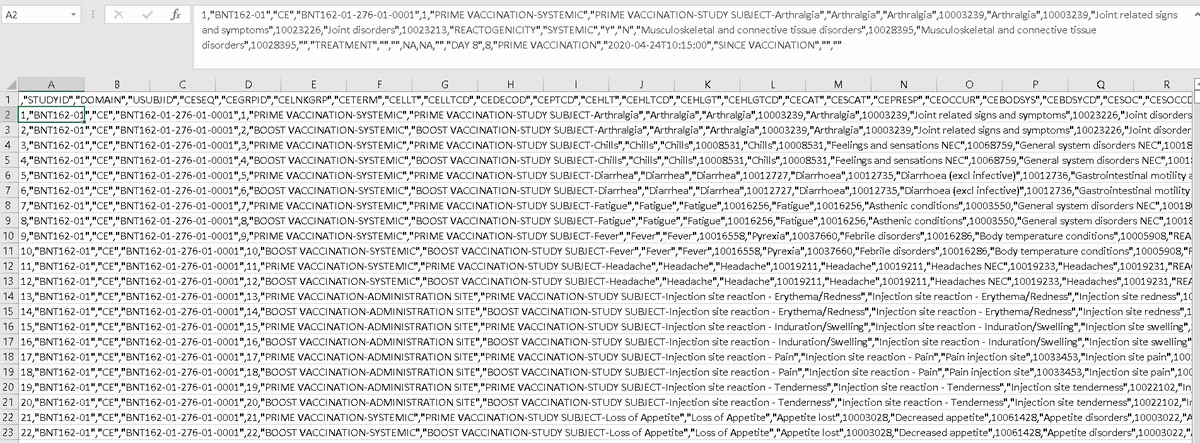
last BNT162-01 file in this batch is S-D-ce.csv
this seems to be another AE list by type, time of onset, prime/boost related, and systemic or injection site related. at first glance there seems to be more AEs listed than made it into the other AE files🤔
this seems to be another AE list by type, time of onset, prime/boost related, and systemic or injection site related. at first glance there seems to be more AEs listed than made it into the other AE files🤔
pdata0916.s3.us-east-2.amazonaws.com/pdocs/110122/1…
the possibly juiciest item in this drop however is this rat fertility/teratogenicity study from june-oct 2020, which has a lot of very questionable results:

the possibly juiciest item in this drop however is this rat fertility/teratogenicity study from june-oct 2020, which has a lot of very questionable results:
https://twitter.com/redpill4me/status/1587545403720859648


so that's it for the brief overview! looking forward to digging into this batch in greater detail, but now i'm off to work 💪💪 #stoptheshots #readthefiles
• • •
Missing some Tweet in this thread? You can try to
force a refresh