holy fucking SHIT. mRNA-1647 is the "bioequivalent" GTMP all of the Moderna regulatory paperwork was done with, and it's been around since 2017. Moderna had mRNA tech ready to go five years ago. @CharlesRixey @joshg99 @TheJikky 
https://twitter.com/JudicialWatch/status/1602773637609803776

the first pages are different versions of the 2017-18 study, here's a neat little freudian slip on page 75. 

analytical procedure is 7 pages of [redacted], but at the end is another set of signatures from 6 july 2017. 



Next up "certificates of analysis" p116, two pages of redactions from 12.4., a single page from 31.5., and another two from 22.6. 






ok i'll have to stop noting the redacted bits because lots of it is. p190 is the first unscarred page in quite a while. 

p217 and following comes some actual data. this might be good to compare to the data there is on the euro version 
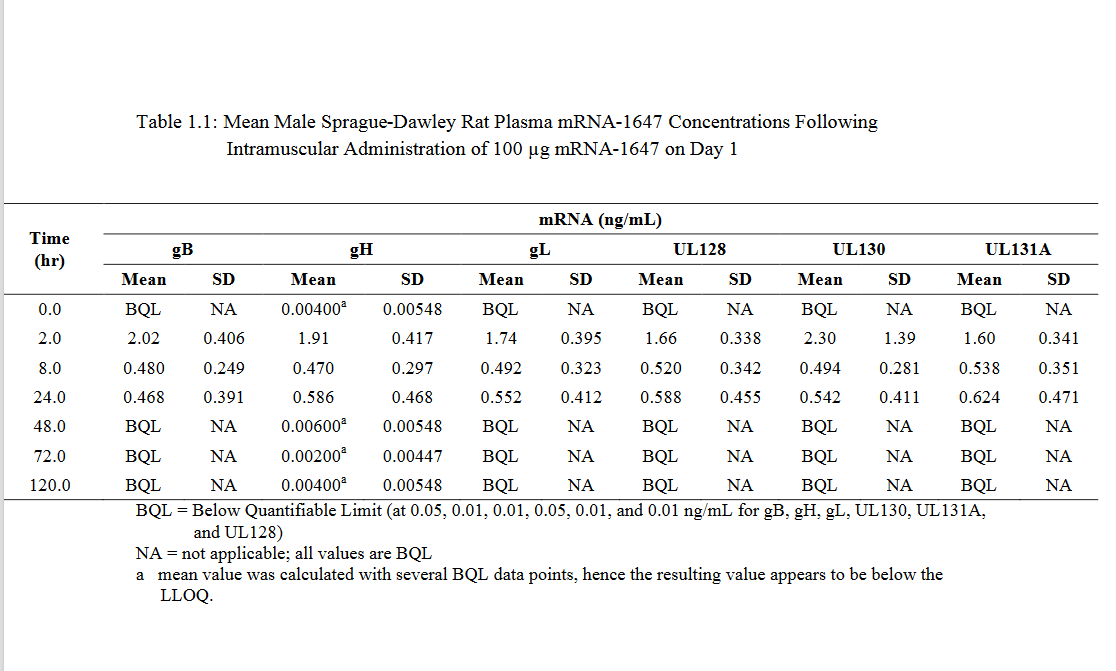
p289 pharmacokinetics. finally a description of what mRNA-1647 actually is: mRNA-1647 contains 6 mRNAs that encode the full-length CMV gB and the pentameric gH/gL/UL128/UL130/UL131A glycoprotein complex. is that hexavalent? sounds a lot like comirnaty LNP-wise 

so this "worked" in instantly euthanized rats five years ago, at least we know that protein was expressed at certain parts of the body, so that's what we've got. wonder what else is coming, this can't be all 

oh the mRNA did not persist longer than three days when you killed all the rats by then? although i haven't gotten to the breeding study yet. 

absolutely incredible. they had this stuff written up since 2017, just waiting on the right pandemic. mrna-1647 could be the placeholder for mrna-1273, covid 2p-S, or maybe mrna-1192, ebola glycoprotein, etc? these screenshots are both p302 



so i've already been reading this for two hours now, gonna take a break.
let me just make this clear, Moderna had more than 2 years do do all kinds of safety studies. the documentation they submitted to the FDA is from a 2017 study.
let me just make this clear, Moderna had more than 2 years do do all kinds of safety studies. the documentation they submitted to the FDA is from a 2017 study.

p306 the moderna FDA submission for mrna-1273 begins. lots of weasely language about using mrna-1647 data 



p308. so they mention lots and lots of studies they did on all these different animals, but then the nonclinical summary looks like this. insane.
oh yeah and there's 5 other moderna vaccines referenced.
oh yeah and there's 5 other moderna vaccines referenced.

p316 the genotoxicity of mRNA vaccines is mainly associated with the LNP formulation and to a lesser extent, the encoded antigen.
well at least they apparently did a genotox study or two. biontech didn't.
well at least they apparently did a genotox study or two. biontech didn't.

they had ralph baric build a mouse-cov-2 !! p317 @TheJikky out to get you from the start @BillyBostickson 

p327 has an overview of all the studies, and 328 a comprehensive cliffnote of the moderna vaccine nonclinical work 





page 330.
"well we found some genotoxicity and some minimal bone marrow damage but because it stays in your arm we think the risk is... low."
the EMA/FDA got this document and decided not to ask for any genotox from pfizer 😬
"well we found some genotoxicity and some minimal bone marrow damage but because it stays in your arm we think the risk is... low."
the EMA/FDA got this document and decided not to ask for any genotox from pfizer 😬

p364 details the genotox studies a bit closer. the in vitros were negative, yet the in vivo was.. positive. they did this one intravenously, but because the human one is IM, its gonna be fine. what the fuck. no idea what micronucleated means and if i open another tab, its over 

few hundred pages of the same (its literally the study and all the redacted stuff as well) then this on page 687: new genotox studies! and repro/fertility. so the first ~x hundred pages were v1 of the submission, and this is an update? 



p691 outlies the repro study. the pups had an immune response, woohey! 6 pups in 4 litters had skeletal aberrations, but they also had antibodies 


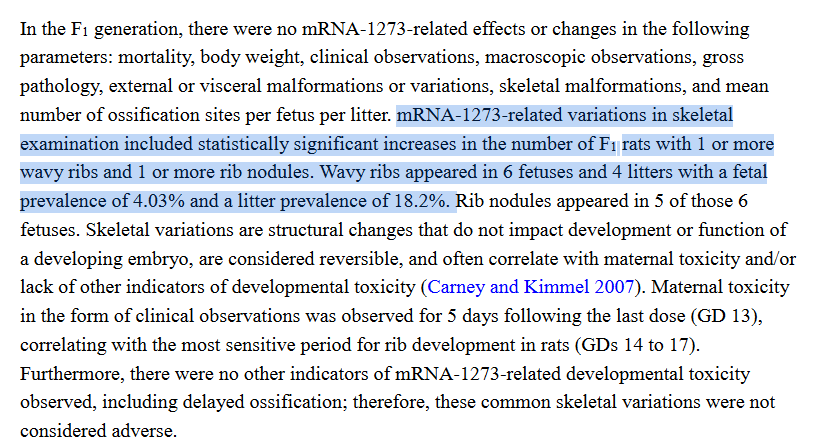
and p695 closes with the "integrated overview and conclusions". two pages further up mentions the end of the phase 3 being close to finished. and that's it. anyone knowledgeable about moderna? was the mRNA-1647 swap known? 



• • •
Missing some Tweet in this thread? You can try to
force a refresh