Argh, this again. Sorry. To understand what is going on here and the actual nature of the question, it really helps to first figure out the pKa of dilute 18OH2 in regular water. Spoiler: it is unambiguously and without choice 15.7. Follow along.
https://twitter.com/J_A_C_S/status/1619296735490183169
For every acid in the universe except water, we can the define the Ka in terms of a ratio of activities that in dilute solution is arbitrarily well approximated by
Ka = [A-][H3O+]/[HA]
If we were figuring out the pKa of any other acid, we would just figure out the
Ka = [A-][H3O+]/[HA]
If we were figuring out the pKa of any other acid, we would just figure out the
ratio of [A-] to [HA] at a given pH, then fill in the values into our equation to find the Ka and pKa. Let's do this with dilute 18OH2 in regular (16OH2) water.
I will make some unnecessary specific assumptions to simplify the math.
Assume pH = 7 and [H3O+] = 1e-7
I will make some unnecessary specific assumptions to simplify the math.
Assume pH = 7 and [H3O+] = 1e-7
Assume [18OH2] = 0.01 M
Now, all we need to figure out the Ka is the concentration of 18OH-.
We know the concentration of 16OH- at neutral pH - it is 1e-7. From this and the simple ratio of 18O and 16O isotopes (concentration or activities are irrelevant for this) the
Now, all we need to figure out the Ka is the concentration of 18OH-.
We know the concentration of 16OH- at neutral pH - it is 1e-7. From this and the simple ratio of 18O and 16O isotopes (concentration or activities are irrelevant for this) the
concentration of 18OH- = 1e-7 x 0.01 M / 55.5 M = 1.802 E-11.
My only assumption has been that the isotope effect is negligible - it will be. There are no other hidden assumptions or choices. Take your time, figure out this number for yourself.
My only assumption has been that the isotope effect is negligible - it will be. There are no other hidden assumptions or choices. Take your time, figure out this number for yourself.
OK, so then the Ka for dilute 18OH2 in regular water is
1e-7 x 1.802 e-11 / 0.01 = 1.802 e-16
and the pKa = -log Ka = 15.7.
Voila.
1e-7 x 1.802 e-11 / 0.01 = 1.802 e-16
and the pKa = -log Ka = 15.7.
Voila.
Assume different pHs or different (but low) [18OH2]. It won't matter, but go ahead, try it.
Ok, so now you are perhaps weirded out that the pKa of regular water in regular water is 14 but the pKa of 18OH2 in regular water, with no isotope effect, is 15.7.
Ok, so now you are perhaps weirded out that the pKa of regular water in regular water is 14 but the pKa of 18OH2 in regular water, with no isotope effect, is 15.7.
Want to be weirded further? Calculate the pKa of dilute regular 16OH2 water in a solution that is otherwise entirely 18OH2. You will find that the pKa of the dilute 16OH2 is, without any choices or sleight of hand, 15.7. So the same water is pKa 14 in 16OH2 but 15.7 in 18OH2!
Ok, so now perhaps you can begin to guess at what is really going on here. It is not a matter of right value versus wrong value, and pretty silly to describe it that way. Rather, it is a matter of changed definitions. For every other acid in water,
we define the denominator in the Ka definition as an activity that is approximated by the concentration in M. For pure water, we choose the activity as simply unity.
Choosing 1 as the activity of a pure solvent is a really good choice, because it simplifies chemistry greatly.
Choosing 1 as the activity of a pure solvent is a really good choice, because it simplifies chemistry greatly.
Think of any equilibrium of ions in water and you quickly realize that you don't know how many waters you should put on each side of the equation. How then do you calculate your Keqs or free energy changes? Not simple. But choosing a solvent activity of 1, the problem goes away.
I have done this for computational studies in other solvents, and it is a really handy trick.
But it is a human choice. One could define the activity of water as 55, for example, and it would make for a much more complicated chemistry in other ways, but it could be done.
But it is a human choice. One could define the activity of water as 55, for example, and it would make for a much more complicated chemistry in other ways, but it could be done.
By the way, Bronsted, by my interpretation, understood all of this.
So, 14 or 15.7? Understand that if you call either a "right value" you are going to run into some paradoxes.
Here is a good one: if we have dilute methanol in water and dilute 18OH2 in water at exactly the same concentration for each, for which one is there more anion present?
Here is a good one: if we have dilute methanol in water and dilute 18OH2 in water at exactly the same concentration for each, for which one is there more anion present?
You can do the math, work it out, there is no ambiguity, at any pH there would be more CH3O– than 18OH-.
At this point, if you are wanting to say that water is more acidic than methanol, don't you feel a bit queazy? 14 feels pretty bad then.
But calculating thermo, 14's better
At this point, if you are wanting to say that water is more acidic than methanol, don't you feel a bit queazy? 14 feels pretty bad then.
But calculating thermo, 14's better
So just maybe, teach students that we are dealing with definitions, that the definition has changed between other acids and water, and that it is ok to change the definition for the purpose at hand. This is done implicitly in computational chemistry all the time, and it's ok.
Since it has been asked about, I thought I should expand slightly on an "assumption" here. I don't see this as an assumption at all - I am just counting - but you might say that I am assuming this: 

Notice that I am not invoking concentration units or activities or equilibrium constants, just the count of the molecules. Another way to say this is that the distribution of 18OH- versus 16OH- is random, reflecting only the relative amounts of 18O versus 16O present.
I don't see any way to get around this or even to fuzzy it up to make it confusing. And once you apply the pictured equation, my calculation for the concentration of 18OH- is set.
Ok, just for fun, let's depart into la-la land and choose a different equation that would make the pKa of dilute 18OH2 be 14 instead of 15.7. This equation would work: 
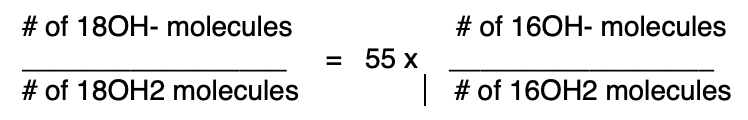
If this last made-up equation were true, then indeed the pKa of dilute 18OH2 in 16OH2 would be 14, not 15.7.
But perhaps you are asking "So how did the number of molecules know about the 55, a number that depends on a human definition of units. Yep, that equation won't hunt.
But perhaps you are asking "So how did the number of molecules know about the 55, a number that depends on a human definition of units. Yep, that equation won't hunt.
• • •
Missing some Tweet in this thread? You can try to
force a refresh





