@TumorBoardTues @drsarahsam 1/24 #TumorBoardTuesday #BreastCancer #OncTwitter
54yo 👩🏻 post-menopausal
HTN
hypothyroidism
FH: aunt with late-onset BC
Germline genetic testing: negative
🔪Dec ‘10 Left lumpectomy + SLNB:
left IDC G2
ER 95%
PgR 10%
HER2-neg (IHC 1+)
Ki67 35%
stage pT2 (25 mm) pN0
Oncotype 32
54yo 👩🏻 post-menopausal
HTN
hypothyroidism
FH: aunt with late-onset BC
Germline genetic testing: negative
🔪Dec ‘10 Left lumpectomy + SLNB:
left IDC G2
ER 95%
PgR 10%
HER2-neg (IHC 1+)
Ki67 35%
stage pT2 (25 mm) pN0
Oncotype 32
@TumorBoardTues @drsarahsam 2/24 #TumorBoardTuesday #BCSM
☢️Jan ‘11: TC x 4 ➡️ XRT
Treatment well tolerated, apart from alopecia, G2 fatigue
Summer ‘11 – started letrozole
🔀 Fall ‘11 – switch to exemestane due to G3 arthralgias ➡️ improvement of symptoms
2016 completed 5 years of Aromatase Inhibitor
☢️Jan ‘11: TC x 4 ➡️ XRT
Treatment well tolerated, apart from alopecia, G2 fatigue
Summer ‘11 – started letrozole
🔀 Fall ‘11 – switch to exemestane due to G3 arthralgias ➡️ improvement of symptoms
2016 completed 5 years of Aromatase Inhibitor
@TumorBoardTues @drsarahsam 3/24 #TumorBoardTuesday #BCSM
Apr ‘21 – Mild abdo discomfort
🩻CT CAP scan:
liver: 5 lesions, max 15 mm
bone: spine & ribs
enlarged mediastinal lymph nodes
🩸: G1 anemia, normal LFTs, no other abnormality
🔬US-guided liver biopsy:
IDC, grade 2, ER 90%, PR 0%, HER2-0, Ki67 25%
Apr ‘21 – Mild abdo discomfort
🩻CT CAP scan:
liver: 5 lesions, max 15 mm
bone: spine & ribs
enlarged mediastinal lymph nodes
🩸: G1 anemia, normal LFTs, no other abnormality
🔬US-guided liver biopsy:
IDC, grade 2, ER 90%, PR 0%, HER2-0, Ki67 25%
@TumorBoardTues @drsarahsam 4/24 #TumorBoardTuesday
🤔 Which 1L systemic treatment would you choose for a post-menopausal patient with metastatic recurrence of HR+ #BreastCancer with the 👆🏽 characteristics from tweets 1-3
🤔 Which 1L systemic treatment would you choose for a post-menopausal patient with metastatic recurrence of HR+ #BreastCancer with the 👆🏽 characteristics from tweets 1-3
@TumorBoardTues @drsarahsam 5/24 #TumorBoardTuesday #BreastCancer
Summer ‘21 – 👩🏻 starts anastrozole + ribociclib 600mg daily 1-21Q28.
Well tolerated, but recurring asymptomatic G4 neutropenia, resolved after dose ⬇️ of ribociclib (400mg 1-21Q28)
Summer ‘21 – 👩🏻 starts anastrozole + ribociclib 600mg daily 1-21Q28.
Well tolerated, but recurring asymptomatic G4 neutropenia, resolved after dose ⬇️ of ribociclib (400mg 1-21Q28)
@TumorBoardTues @drsarahsam 6/24 #TumorBoardTuesday #BreastCancer
Fall ‘21 CT CAP scan:
partial response of liver and lymph nodal lesions
increased sclerosis of bone mets
🧐 In which situation would you have preferred to use 1L chemotherapy instead of endocrine therapy?
Fall ‘21 CT CAP scan:
partial response of liver and lymph nodal lesions
increased sclerosis of bone mets
🧐 In which situation would you have preferred to use 1L chemotherapy instead of endocrine therapy?
@TumorBoardTues @drsarahsam 7/24 #TumorBoardTuesday #BreastCancer
🧐 In which situation would you have preferred palbociclib or abemaciclib?
🧐 In which situation would you have preferred palbociclib or abemaciclib?
@TumorBoardTues @drsarahsam 8/24 #TumorBoardTuesday
👩🏻🏫Mini tweetorial 1👨🏻🏫
📌Early stage, HR+ #BreastCancer
🔹Most common subtype: ~ 70% of cases
🔹historically treated with endocrine tx & chemotherapy
🔹Gene signatures (ex OncotypeDX) allow to estimate benefit of chemo & aid clinical decision making
👩🏻🏫Mini tweetorial 1👨🏻🏫
📌Early stage, HR+ #BreastCancer
🔹Most common subtype: ~ 70% of cases
🔹historically treated with endocrine tx & chemotherapy
🔹Gene signatures (ex OncotypeDX) allow to estimate benefit of chemo & aid clinical decision making
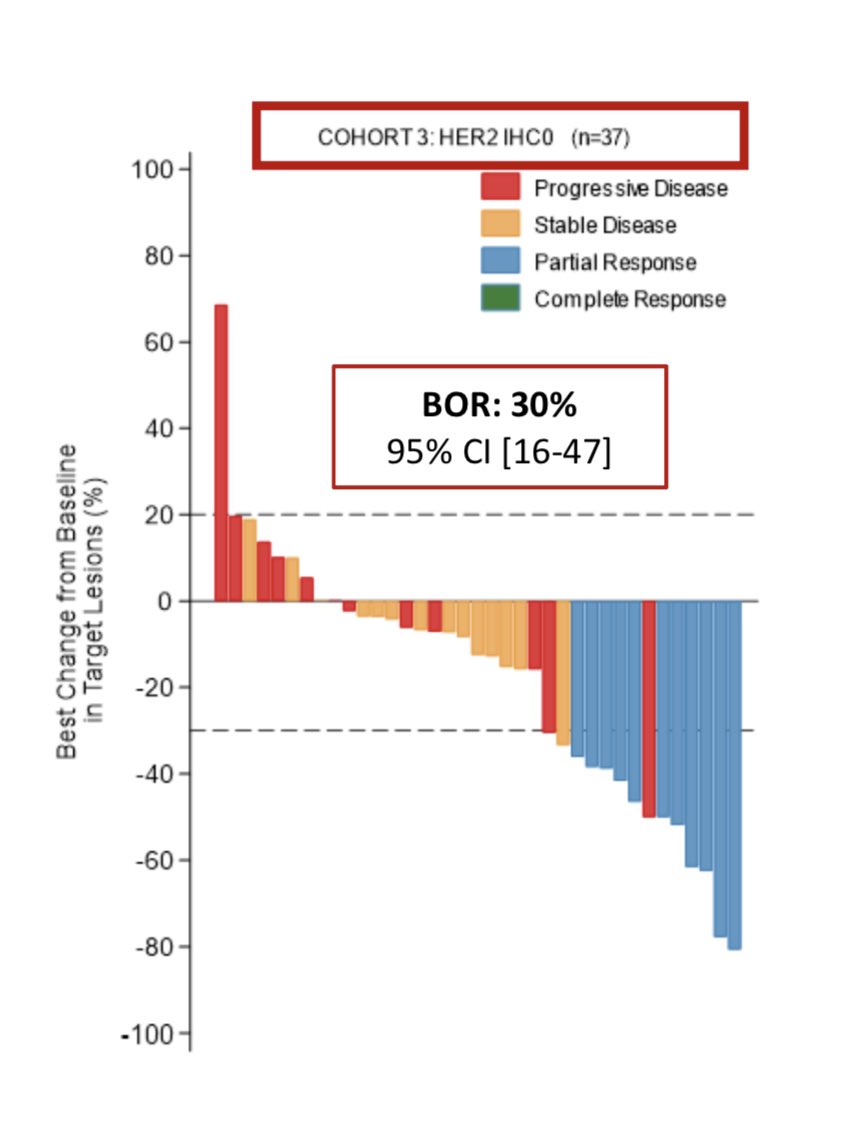
@TumorBoardTues @drsarahsam 9/24 #TumorBoardTuesday #BCSM
👩🏻🏫Mini tweetorial 2👨🏻🏫
📌Adjuvant abemaciclib - CDK4/6 inhibitor
📍Tested in monarchE phase 3 trial
📍Last update of the study: 2 years of adjuvant abema added to ET led to ⬇️ by 33% in risk of recurrence compared with adjuvant ET alone
👩🏻🏫Mini tweetorial 2👨🏻🏫
📌Adjuvant abemaciclib - CDK4/6 inhibitor
📍Tested in monarchE phase 3 trial
📍Last update of the study: 2 years of adjuvant abema added to ET led to ⬇️ by 33% in risk of recurrence compared with adjuvant ET alone

@TumorBoardTues @drsarahsam 10/24 #TumorBoardTuesday #BCSM
👩🏻🏫Mini tweetorial 3👨🏻🏫
📌Adjuvant Palbociclib - CDK4/6 inhibitor
📍Tested in the adjuvant setting
📍Failed to improve outcomes in 2 phase 3 trials (✨PALLAS & ✨PENELOPE-B).
📍Phase 3 trial of adjuvant ribociclib ongoing (✨NATALEE)
✍🏼
👩🏻🏫Mini tweetorial 3👨🏻🏫
📌Adjuvant Palbociclib - CDK4/6 inhibitor
📍Tested in the adjuvant setting
📍Failed to improve outcomes in 2 phase 3 trials (✨PALLAS & ✨PENELOPE-B).
📍Phase 3 trial of adjuvant ribociclib ongoing (✨NATALEE)
✍🏼
@TumorBoardTues @drsarahsam 11/24 #TumorBoardTuesday
👩🏻🏫Mini tweetorial 4👨🏻🏫
📌CDK 4/6 inhibitor
🔹1L metastatic setting, adding CDK4/6i to endocrine treatment has demonstrated significant & comparable improvements in PFS across phase 3 trials of 3 different agents (palbociclib, ribociclib, abemaciclib)
👩🏻🏫Mini tweetorial 4👨🏻🏫
📌CDK 4/6 inhibitor
🔹1L metastatic setting, adding CDK4/6i to endocrine treatment has demonstrated significant & comparable improvements in PFS across phase 3 trials of 3 different agents (palbociclib, ribociclib, abemaciclib)

@TumorBoardTues @drsarahsam 12/24 #TumorBoardTuesday
👩🏻🏫Mini tweetorial 5👨🏻🏫
📌CDK 4/6 inhibitor
Despite similar PFS efficacy, three CDK4/6 inhibitors differ in toxicity profile
💊Palbociclib: more neutropenia
💊Ribociclib: more ⬆️ LFT
🫧can cause prolong QTc
💊Abemaciclib: more GI tox, ⬇️ neutropenia
👩🏻🏫Mini tweetorial 5👨🏻🏫
📌CDK 4/6 inhibitor
Despite similar PFS efficacy, three CDK4/6 inhibitors differ in toxicity profile
💊Palbociclib: more neutropenia
💊Ribociclib: more ⬆️ LFT
🫧can cause prolong QTc
💊Abemaciclib: more GI tox, ⬇️ neutropenia
@TumorBoardTues @drsarahsam 13/24 #TumorBoardTuesday
👩🏻🏫Mini tweetorial 6👨🏻🏫
📌CDK 4/6 inhibitors
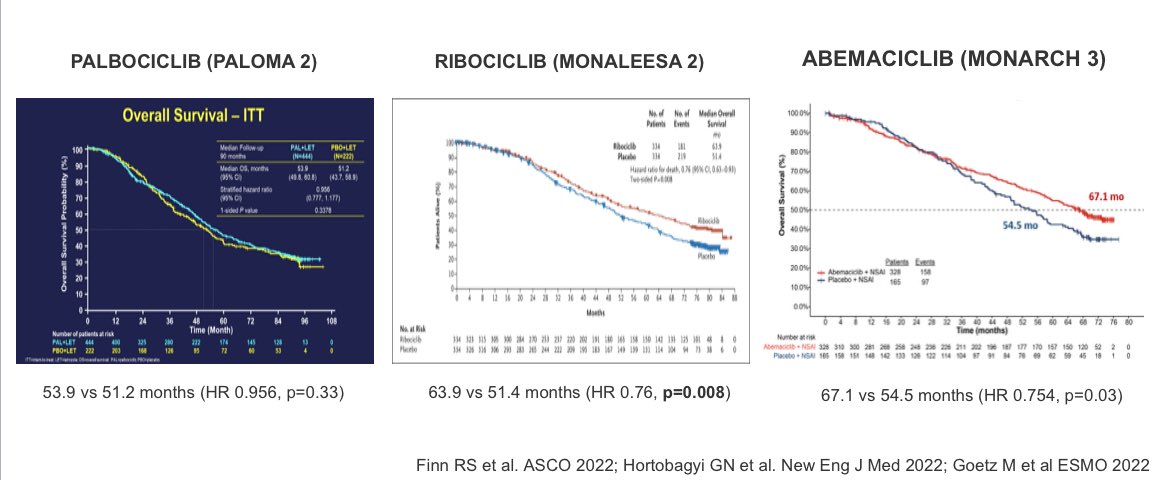
OS results p3 recently reported
✨PALOMA2 (palbociclib):👎🏽 significant OS advantage
✨MONALEESA2 (ribociclib):👍🏽significant OS advantage
✨MONARCH3 (abemaciclib): data immature, non-significant OS improvement
👩🏻🏫Mini tweetorial 6👨🏻🏫
📌CDK 4/6 inhibitors
OS results p3 recently reported
✨PALOMA2 (palbociclib):👎🏽 significant OS advantage
✨MONALEESA2 (ribociclib):👍🏽significant OS advantage
✨MONARCH3 (abemaciclib): data immature, non-significant OS improvement
@TumorBoardTues @drsarahsam 14/24 #TumorBoardTuesday
👩🏻🏫Mini tweetorial 7👨🏻🏫
🧐How to choose which CDK4/6i for 1L?
📍Ribociclib
📍Palbociclib
📍Abemaciclib
✅Shared decision
🫧available data
🫧comorbidities
🫧patient preference
✅Given OS advantage
🫧absence of contraindications
➡️prioritize ribociclib⬅️
👩🏻🏫Mini tweetorial 7👨🏻🏫
🧐How to choose which CDK4/6i for 1L?
📍Ribociclib
📍Palbociclib
📍Abemaciclib
✅Shared decision
🫧available data
🫧comorbidities
🫧patient preference
✅Given OS advantage
🫧absence of contraindications
➡️prioritize ribociclib⬅️
@TumorBoardTues @drsarahsam 15/24 #TumorBoardTuesday
👩🏻🏫Mini tweetorial 8👨🏻🏫
⚠️Ribociclib⚠️
🫀Avoid combination with tamoxifen, given increased risk of QTc prolongation with the combo‼️
👩🏻🏫Mini tweetorial 8👨🏻🏫
⚠️Ribociclib⚠️
🫀Avoid combination with tamoxifen, given increased risk of QTc prolongation with the combo‼️
@TumorBoardTues @drsarahsam @aydah_alawadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova
@TumorBoardTues @drsarahsam @Aydah_AlAwadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova 16/24 #TumorBoardTuesday
Back to our case🔎
Feb ‘23: multifocal progress of disease
new small liver mets
multiple new asymptomatic bone mets (PFS= 17 months)
ECOG PS: 1, no signs of visceral crisis
NGS on ctDNA: ESR1 mutation (Y537S)
🤔What treatment opts available in 2L?
Back to our case🔎
Feb ‘23: multifocal progress of disease
new small liver mets
multiple new asymptomatic bone mets (PFS= 17 months)
ECOG PS: 1, no signs of visceral crisis
NGS on ctDNA: ESR1 mutation (Y537S)
🤔What treatment opts available in 2L?
@TumorBoardTues @drsarahsam @Aydah_AlAwadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova 17/24 #TumorBoardTuesday
📌Choosing 2L treatment
Absence of PIK3CA mutations excludes alpelisib as an option.
Presence of an ESR1 mutation suggests a SERD may be beneficial
Fulvestrant is an IM 💉SERD historically utilized as SOC for patients progressing to tamoxifen or AI
📌Choosing 2L treatment
Absence of PIK3CA mutations excludes alpelisib as an option.
Presence of an ESR1 mutation suggests a SERD may be beneficial
Fulvestrant is an IM 💉SERD historically utilized as SOC for patients progressing to tamoxifen or AI
@TumorBoardTues @drsarahsam @Aydah_AlAwadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova 18/24 #TumorBoardTuesday #BreastCancer
📌Choosing 2L treatment
🕵🏻♀️Recent trials have shown poor outcomes with fulvestrant monotherapy after CDK4/6 inhibitors.
📚 e.g. VERONICA trial (PFS 2 months): aacrjournals.org/clincancerres/…
📌Choosing 2L treatment
🕵🏻♀️Recent trials have shown poor outcomes with fulvestrant monotherapy after CDK4/6 inhibitors.
📚 e.g. VERONICA trial (PFS 2 months): aacrjournals.org/clincancerres/…

@TumorBoardTues @drsarahsam @Aydah_AlAwadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova 19/24 #TumorBoardTuesday
📌Fulvestrant combos
✨MAINTAIN: + ribociclib (after progress to other CDK4/6i): sig PFS advantage over fulv mono
🔹Await p3 post CDK4/6
✨PrE0102: + everolimus: sig PFS advantage over fulv mono
👏🏽phase 3 novel oral SERD data have led to new approval
📌Fulvestrant combos
✨MAINTAIN: + ribociclib (after progress to other CDK4/6i): sig PFS advantage over fulv mono
🔹Await p3 post CDK4/6
✨PrE0102: + everolimus: sig PFS advantage over fulv mono
👏🏽phase 3 novel oral SERD data have led to new approval
@TumorBoardTues @drsarahsam @Aydah_AlAwadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova 20/24 #TumorBoardTuesday
✨EMERALD✨
📍phase 3
📍oral SERD elacestrant outperformed SoC endocrine treatment (AI or Fulvestrant) in endocrine-refractory MBC
📍benefit enhanced in ESR1-mutant #BreastCancer & in patients who have received > 12 months of prior CDK4/6 inhibitors
✨EMERALD✨
📍phase 3
📍oral SERD elacestrant outperformed SoC endocrine treatment (AI or Fulvestrant) in endocrine-refractory MBC
📍benefit enhanced in ESR1-mutant #BreastCancer & in patients who have received > 12 months of prior CDK4/6 inhibitors

@TumorBoardTues @drsarahsam @Aydah_AlAwadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova 21/24 #TumorBoardTuesday #BreastCancer
✨EMERALD✨
🎉 Based on data, elacestrant was approved on Jan 27, 2023 for treatment of patients with ESR1-mutant HR+ breast cancer who have progressed to at least 1L of endocrine treatment
📚 ascopubs.org/doi/full/10.12…
✨EMERALD✨
🎉 Based on data, elacestrant was approved on Jan 27, 2023 for treatment of patients with ESR1-mutant HR+ breast cancer who have progressed to at least 1L of endocrine treatment
📚 ascopubs.org/doi/full/10.12…
@TumorBoardTues @drsarahsam @Aydah_AlAwadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova 22/24 #TumorBoardTuesday #BreastCancer
✨CAPItello291✨
Ph3 of fulvestrant with/without Akt-inhibitor capivasertib 👉 addition of biologic ⬆️PFS!
Enhanced benefit in patients with alterations in PIK3CA/Akt/mTOR pathway (~40%)
but
🤞Potential benefit in pts without alterations
✨CAPItello291✨
Ph3 of fulvestrant with/without Akt-inhibitor capivasertib 👉 addition of biologic ⬆️PFS!
Enhanced benefit in patients with alterations in PIK3CA/Akt/mTOR pathway (~40%)
but
🤞Potential benefit in pts without alterations

@TumorBoardTues @drsarahsam @Aydah_AlAwadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova 23/24 #TumorBoardTuesday #BreastCancer
🎉Multiplicity of potential treatment options are becoming available for patients progressing to 1L ET + CDK4/6-inh
🎉expected to improve outcomes in near future for this large population of patients
Check this algorithm by @drsarahsam 👇
🎉Multiplicity of potential treatment options are becoming available for patients progressing to 1L ET + CDK4/6-inh
🎉expected to improve outcomes in near future for this large population of patients
Check this algorithm by @drsarahsam 👇

@TumorBoardTues @drsarahsam @Aydah_AlAwadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova 24/24 #TumorBoardTuesday
👩🏻Given the presence of an ESR1 mutation and exposure to >12 months of ribociclib, elacestrant was chosen as 2L treatment.
First 4 weeks: good tolerance, G1 nausea
🩻Scans in 4 weeks
🤞🏽Fingers crossed!!
👩🏻Given the presence of an ESR1 mutation and exposure to >12 months of ribociclib, elacestrant was chosen as 2L treatment.
First 4 weeks: good tolerance, G1 nausea
🩻Scans in 4 weeks
🤞🏽Fingers crossed!!
@TumorBoardTues @drsarahsam @Aydah_AlAwadhi @Dr_AmerZeidan @dr_khaledamiri @MariamBird @DrMAttiaE @jobybabyjolly @LeandroJonatad1 @kyutibu @alshamsi2000 @haldhanhani86 @IAbuGhiedaMD @Htyfoor @jamecancerdoc @gandhi_shipra @RayMacCuUladh @draalicefrancis @sflomos @JenniferPlichta @Marie_Tsvetkova #PostTest Q1️⃣ #TumorBoardTuesday
👉🏽#CME Eval integrityce.com/tbtEval
🤔@drsarahsam @PTarantinoMD taught us CDK 4/6i
🧐Which 1L would U pick for 54yo👩🏻🦱postmen
HTN, hypothyroid
met recur liver, spine, ribs of ER+/HER2- (IHC 0) BC prev tx TC & XRT, letrozole, exemestane, & 5yr of AI
👉🏽#CME Eval integrityce.com/tbtEval
🤔@drsarahsam @PTarantinoMD taught us CDK 4/6i
🧐Which 1L would U pick for 54yo👩🏻🦱postmen
HTN, hypothyroid
met recur liver, spine, ribs of ER+/HER2- (IHC 0) BC prev tx TC & XRT, letrozole, exemestane, & 5yr of AI
• • •
Missing some Tweet in this thread? You can try to
force a refresh