I congratulate the team who worked diligently to produce the PANGEA model to predict myeloma risk
This is the first "dynamic" model that assesses risks, and in my opinion is a step forward from existing models.
Some thoughts in 🧵
#mmsm
thelancet.com/journals/lanha…
This is the first "dynamic" model that assesses risks, and in my opinion is a step forward from existing models.
Some thoughts in 🧵
#mmsm
thelancet.com/journals/lanha…
Firstly, I commend the team for choosing a trainee (@anniencowan ) as a first author. So inspirational. I saw that for the PROMISE study too, @HabibElKhoury was first author).
This is the way 👏
This is the way 👏
I also admit I am not a statistician, and this was a very technical read. The results and methods are not an easy read for a clinician. I will focus my comments on the clinical aspect of things, because some of the stats in this manuscript are simply over my head.
⭐️Smoldering myeloma diagnosed today in 2023 is AFTER rigorous advanced imaging that has excluded bone dis/MM (ideally a myeloma MRI).
How many patients in this cohort had advanced imaging like a myeloma MRI or PET/CT done?
This is critically important, but couldnt find this.
How many patients in this cohort had advanced imaging like a myeloma MRI or PET/CT done?
This is critically important, but couldnt find this.
Why is this important
If not done possible that patients in smoldering cohort actually had MM.
This is also why older data (such as Spanish trial 👇not applicable to smoldering of today)
pubmed.ncbi.nlm.nih.gov/23902483/
If not done possible that patients in smoldering cohort actually had MM.
This is also why older data (such as Spanish trial 👇not applicable to smoldering of today)
pubmed.ncbi.nlm.nih.gov/23902483/
⭐️How many patients progressed to SLiM criteria, versus actual CRAB criteria?
This is also critically important. In recent studies, the progression rate of SLiM criteria is turning out to be much lower than we originally thought.
See this study below:
ascopubs.org/doi/abs/10.120…
This is also critically important. In recent studies, the progression rate of SLiM criteria is turning out to be much lower than we originally thought.
See this study below:
ascopubs.org/doi/abs/10.120…
⭐️How much of this cohort (those with smoldering) was rigorously screened for SLiM criteria at entry? Again, that information- seemed to be lacking in the manuscript and is important context.
Ideally, Table 1 should have all of this information.
Ideally, Table 1 should have all of this information.
⭐️What about genomic data? We arent there yet, but genomic signatures that predict disease progression with near-certainty may be the future. Genomic models were not incorporated in this model, but may be in the future?
⭐️FISH information is hard to get for patients with MGUS (low plasma cell volume), but was missing for most patients in this dataset, and did not make it to the final publicly accessible model. 
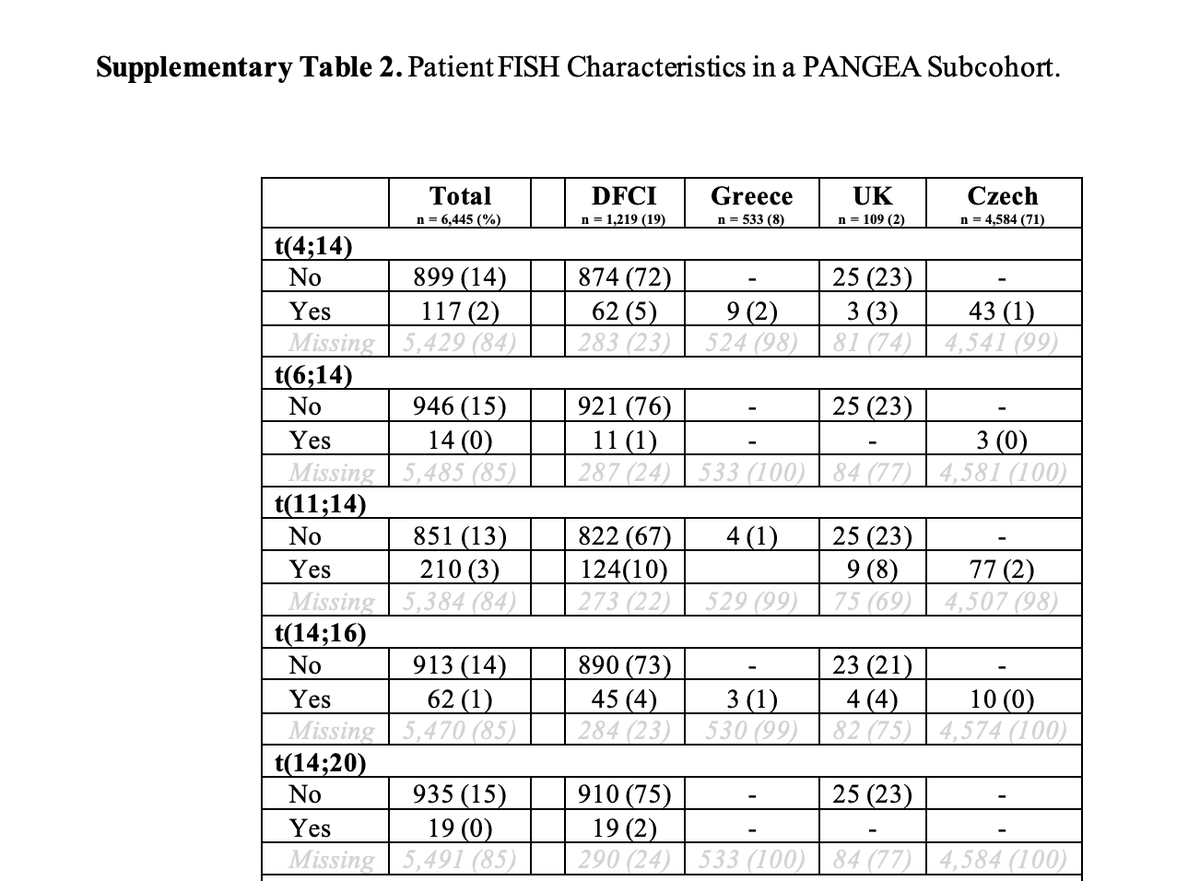
⭐️Interesting that sig minority of patients from DFCI cohort (18%!) but not the other cohorts were censored because treatment was started early. This speaks to different treatment patterns (perhaps DFCI enrolled the highest risk on clinical trials). May have affected this model.
Now the good stuff-
I am no expert at C-statistics, but a higher value relative to existing models such as 2/20/20 tells us this model appears to be predicting better than previous models (and better than chance- which is a C-statistic of 0.5).
I am no expert at C-statistics, but a higher value relative to existing models such as 2/20/20 tells us this model appears to be predicting better than previous models (and better than chance- which is a C-statistic of 0.5).

The website is publicly accessible and very easy to use.
I tried putting in information for a few patients and was happy with the user friendly way it worked.
Lets show an example below:
pangeamodels.org
I tried putting in information for a few patients and was happy with the user friendly way it worked.
Lets show an example below:
pangeamodels.org
62 yr old male, diagnosed with smoldering June 2022.
Bmbx= 30% PC
M spike=1.45
Ratio=22
Cr=0.8
Hb=11.1
Gain1q on FISH
Hb Jan 22= 12.1
Patient does have 2/3 of the 2/20/20 risk features!
On IMWG model, 2 year risk-26%
On PANGEA, 2 year risk=10.7%

Bmbx= 30% PC
M spike=1.45
Ratio=22
Cr=0.8
Hb=11.1
Gain1q on FISH
Hb Jan 22= 12.1
Patient does have 2/3 of the 2/20/20 risk features!
On IMWG model, 2 year risk-26%
On PANGEA, 2 year risk=10.7%
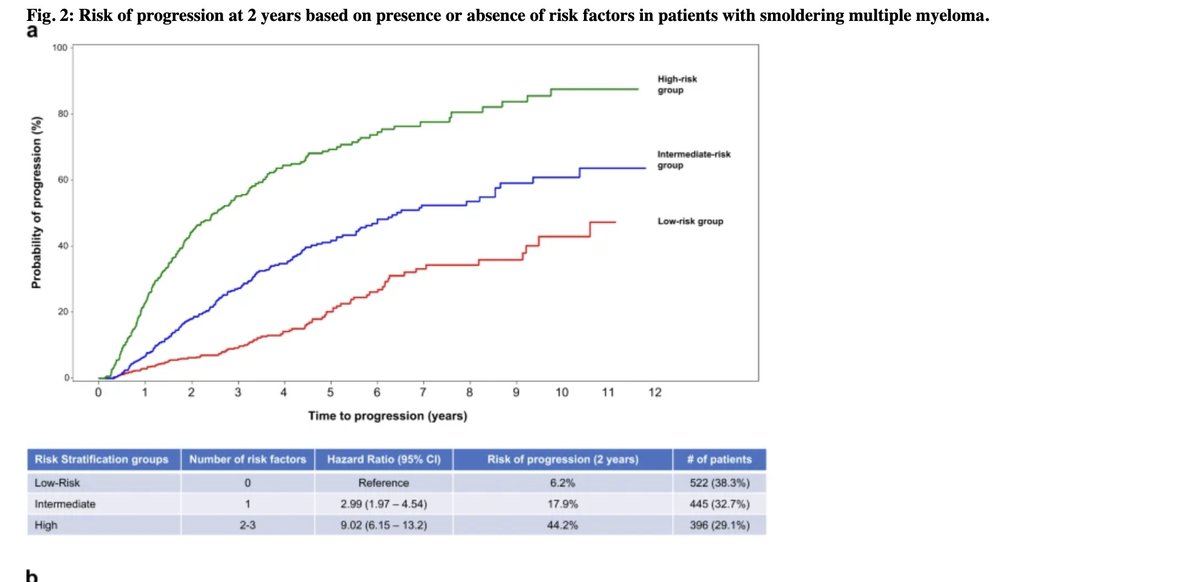
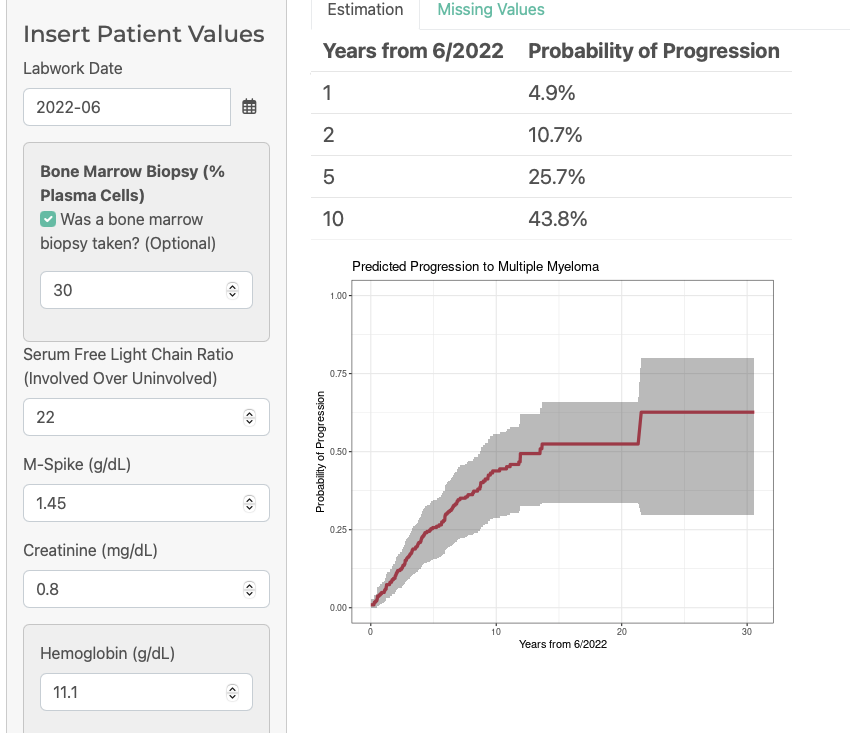
I was surprised that the model did not account for changes in M spikes and light chain ratio over time. It did account for hemoglobin. As a clinician, would have been nice to see model account for those changes too.
So in summary
⭐️This is a useful model that I will be using in clinic
⭐️Uncertainty remains whether it is applicable to modern cohort with myeloma excluded via advanced imaging
⭐️Not a perfect predictor of risk, and I wouldnt routinely offer treatment to high-risk on this model
⭐️This is a useful model that I will be using in clinic
⭐️Uncertainty remains whether it is applicable to modern cohort with myeloma excluded via advanced imaging
⭐️Not a perfect predictor of risk, and I wouldnt routinely offer treatment to high-risk on this model
• • •
Missing some Tweet in this thread? You can try to
force a refresh