The carfilzomib (K) versus bortezomib (V) saga for newly diagnosed myeloma.
An educational, historical and philosophical thread.
#mmsm
An educational, historical and philosophical thread.
#mmsm
V was first approved by the FDA in 2004.
K approved in 2013.
As opposed to V, K is an "irreversible" PI- and is much more active against myeloma cell lines compared to V
(note=pre-clinical efficacy is NOT clinical efficacy- melflufen was 50x more potent than melphalan 🧐!)
K approved in 2013.
As opposed to V, K is an "irreversible" PI- and is much more active against myeloma cell lines compared to V
(note=pre-clinical efficacy is NOT clinical efficacy- melflufen was 50x more potent than melphalan 🧐!)

For a me-too drug to be useful it must offer at least one of the following features:
1) It works when original drug stops working
2) It has more "clinical" efficacy (not just againt cell lines) than original drug
3) It is safer/has different adverse events than the original drug
1) It works when original drug stops working
2) It has more "clinical" efficacy (not just againt cell lines) than original drug
3) It is safer/has different adverse events than the original drug
This is why in MM- pomalidomide and carfilzomib represent true advances and new "options" for patients- they have at least one of the above.
Isatuximab, despite being a great drug and probably at least as effective/safe as daratumumab- doesnt have any of the above over dara
Isatuximab, despite being a great drug and probably at least as effective/safe as daratumumab- doesnt have any of the above over dara
In relapsed/refractory myeloma in a trial of which 54% of patients had previously received bortezomib, the use of carfilzomib compared to bortezomib led to improved PFS and overall survival (there was no cross-over to carfilzomib for the bortezomib arm).
thelancet.com/journals/lanon…
thelancet.com/journals/lanon…
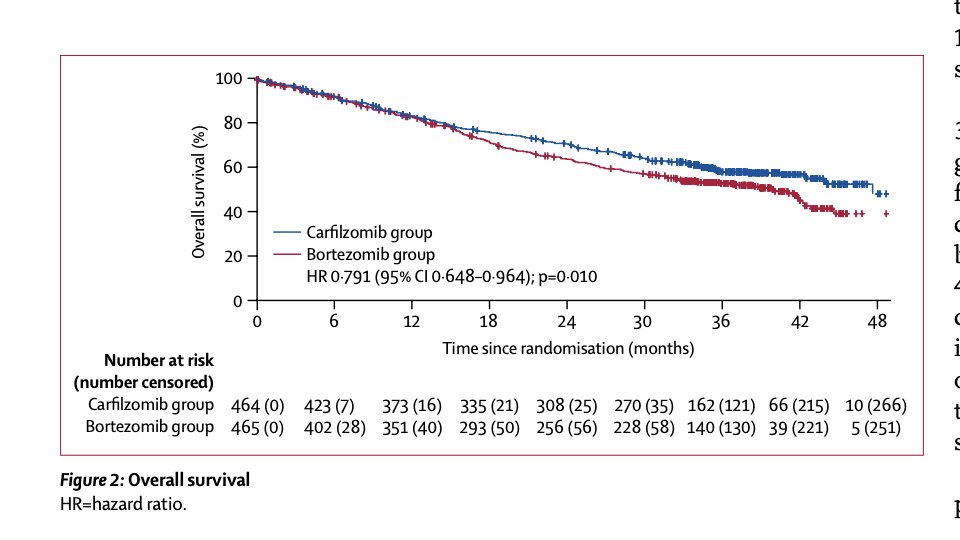
Now this trial taught us that K is active in patients with previous V "exposure"- and is a better option compared to reusing V for relapsed myeloma.
Tragic sidenote- despite this trial being published in 2015, bortezomib-dex continued to used as control arm for many years.
Tragic sidenote- despite this trial being published in 2015, bortezomib-dex continued to used as control arm for many years.
But this trial did not establish that K is better than V for newly diagnosed MM.
Two trials attempted to do that.
Lets go over them.
Two trials attempted to do that.
Lets go over them.
Trial 1: CLARION
Carfilzomib/melphalan/prednisone versus bortezomib/melphalan/prednisone for transplant ineligible disease.
Virtually overlapping survival curves. Negative trial.
In high-risk (4;14, t(14;16), or del 17p), no benefit either👇
pubmed.ncbi.nlm.nih.gov/30819926/

Carfilzomib/melphalan/prednisone versus bortezomib/melphalan/prednisone for transplant ineligible disease.
Virtually overlapping survival curves. Negative trial.
In high-risk (4;14, t(14;16), or del 17p), no benefit either👇
pubmed.ncbi.nlm.nih.gov/30819926/
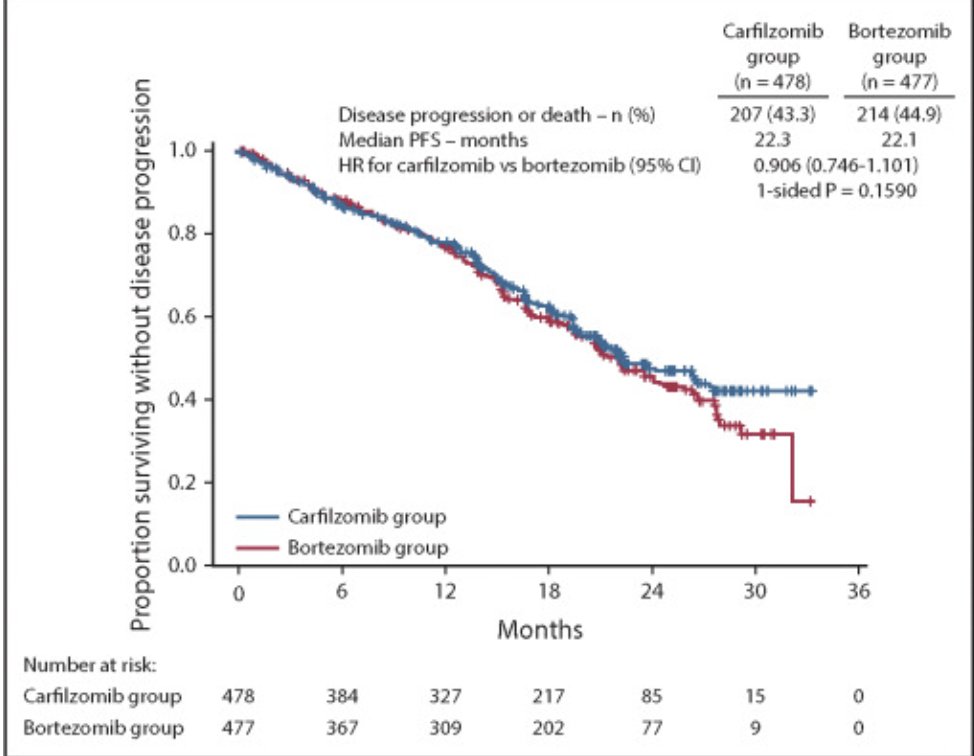
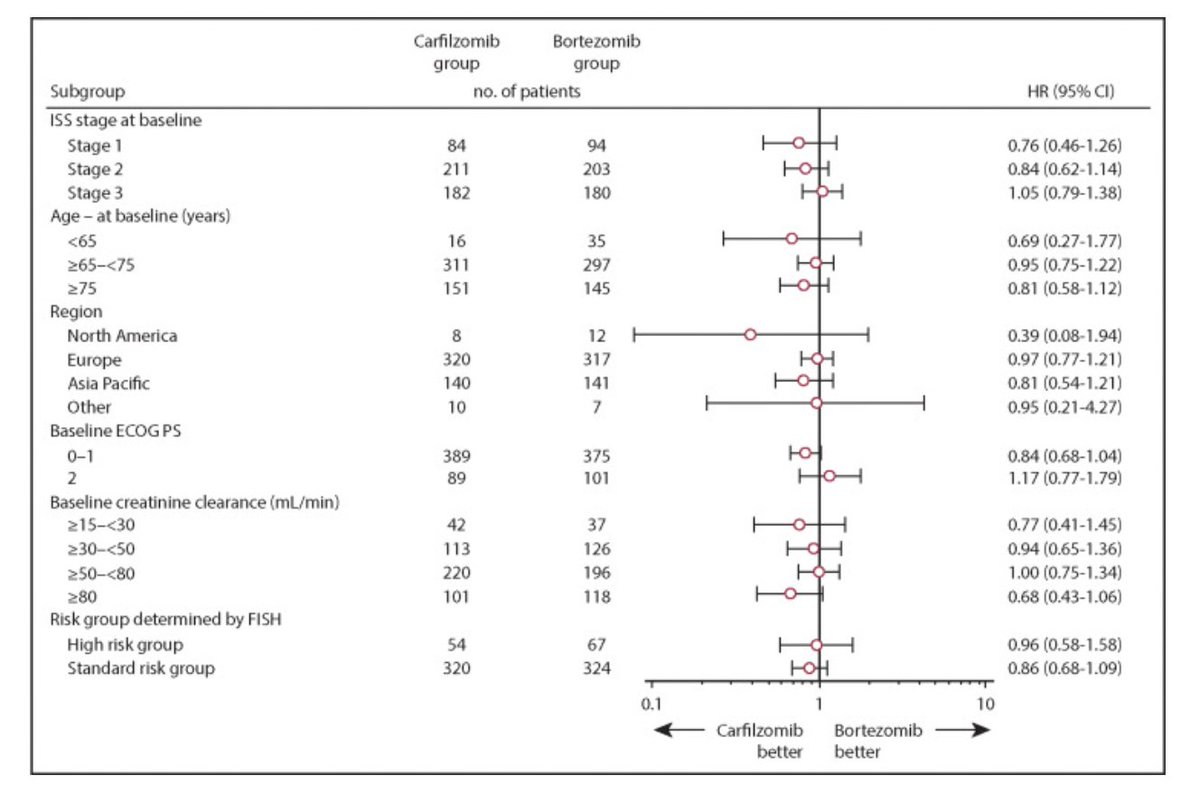
Trial 2: ENDURANCE
Bortezomib/lenalidomide/dex versus carfilzomib/lenalidomide/dex for those not intended for early transplant.
Again, virtually overlapping survival curves. Negative trial.
thelancet.com/article/S1470-…
Bortezomib/lenalidomide/dex versus carfilzomib/lenalidomide/dex for those not intended for early transplant.
Again, virtually overlapping survival curves. Negative trial.
thelancet.com/article/S1470-…

What about high-risk?
In ENDURANCE, t4;14 and Gain/Amp 1q were enrolled, but not t14;16, t14;20 or del17p.
Those patients were enrolled on a parallel SWOG trial comparing elo-VRD to VRD.
What about the subset of patients with 1q abnormalities or 4;14?
In ENDURANCE, t4;14 and Gain/Amp 1q were enrolled, but not t14;16, t14;20 or del17p.
Those patients were enrolled on a parallel SWOG trial comparing elo-VRD to VRD.
What about the subset of patients with 1q abnormalities or 4;14?
Data gets noisy when we go into these small subgroups, especially when overall trial was negative.
For gain1q, K appeared better.
For amp1q, V appeared better (hard to biologically explain).
For 4;14, V actually appeared better too!
For gain1q, K appeared better.
For amp1q, V appeared better (hard to biologically explain).
For 4;14, V actually appeared better too!
So in summary, two randomized trials showed no convincing benefit for K compared to V for newly diagnosed disease, including those with high-risk disease.
When we have high-quality randomized data for a specific question, observational studies and single arm less relevant.
When we have high-quality randomized data for a specific question, observational studies and single arm less relevant.
Nevertheless, many single-arm studies have shown great efficacy of KRd.
Throughout oncology we have seen that single-arm Phase 2 studies show improved activity against historical controls, and that this effect diminishes in randomized trials.
Examples of 1 arm KRD studies👇

Throughout oncology we have seen that single-arm Phase 2 studies show improved activity against historical controls, and that this effect diminishes in randomized trials.
Examples of 1 arm KRD studies👇

However, even in retrospective observational data- the MD Anderson group led by @mahrefat showed that KRd no better than VRd for newly diagnosed high-risk disease.
nature.com/articles/s4140…
nature.com/articles/s4140…

After reading all of the above, it is abundantly clear to me that there doesnt appear to be a benefit with K compared to K for newly diagnosed disease.
And that in the randomized trials, even for high-risk disease, there appears to be no benefit.
One more key point though.
And that in the randomized trials, even for high-risk disease, there appears to be no benefit.
One more key point though.
Even if there was a minor PFS benefit with carfilzomib, given that carfilzomib is a highly effective agent for relapsed disease, it is unlikely that long-term outcomes would truly be better with K up-front (since you could use carfilzomib later and get same/more mileage).
There was a new pre-print just released as well, that yet again compares K to V. Whereas this has not been peer-reviewed (I would love to peer review this), this too must be weighed against the convincing randomized data that (IMO) has conclusively answered this question.
Link to pre-print:
pubmed.ncbi.nlm.nih.gov/36865246/
I will refrain from a thorough peer-review, but I only wish to draw attention to the survival curve and how it potentially indicates residual bias/confounding.
pubmed.ncbi.nlm.nih.gov/36865246/
I will refrain from a thorough peer-review, but I only wish to draw attention to the survival curve and how it potentially indicates residual bias/confounding.
Lets first look at a study we already discussed above.
K vs V for relapsed disease. K was ACTUALLY better. Look at the KM curve for PFS. A clear benefit, but over time- consistent with an activity of preventing some progressions "over-time".
Curves initially overlap.
K vs V for relapsed disease. K was ACTUALLY better. Look at the KM curve for PFS. A clear benefit, but over time- consistent with an activity of preventing some progressions "over-time".
Curves initially overlap.

Now let's look at K vs V in this pre-print.
There is a dramatic early separation of curves. This indicates most probably residual confounding rather than a true ability of K to prevent early progressions.
(something that was not seen in any of the randomized trials).
There is a dramatic early separation of curves. This indicates most probably residual confounding rather than a true ability of K to prevent early progressions.
(something that was not seen in any of the randomized trials).

Key point:
The reason why we intensely debate K versus V and not pom versus len, is because K is made by a different company compared to V.
For all we know, pom maybe better than len, but we have not been financially incentivized to study it since celgene makes both.
The reason why we intensely debate K versus V and not pom versus len, is because K is made by a different company compared to V.
For all we know, pom maybe better than len, but we have not been financially incentivized to study it since celgene makes both.
That sums up this thread. Hopefully adds key context to the debate. Thanks for reading!
@AaronGoodman33 @VPrasadMDMPH @rajshekharucms @HadidiSamer @TMSchmidtMD @Eddie_Cliff @dgermain21
END.
@AaronGoodman33 @VPrasadMDMPH @rajshekharucms @HadidiSamer @TMSchmidtMD @Eddie_Cliff @dgermain21
END.
• • •
Missing some Tweet in this thread? You can try to
force a refresh