I’ve refrained from commenting on metagenomics of environmental samples from Huanan Seafood Market, since media coverage preceded description of data or analysis.
Data still not available, but there is now enough written down to offer partially informed assessment.
Data still not available, but there is now enough written down to offer partially informed assessment.
TLDR is I agree w WHO’s @DrTedros (
These data don’t tell us how pandemic began, but every bit of information helps.
Here is what I glean from the available analyses by the two groups w access.
https://twitter.com/WHO/status/1636704883091857409):
These data don’t tell us how pandemic began, but every bit of information helps.
Here is what I glean from the available analyses by the two groups w access.
First analysis is pre-print Chinese CDC posted over year ago (Feb-25-2022) that describes sampling animals & environment at Huanan Market: researchsquare.com/article/rs-137…
It is moving thru peer review at glacial pace: @researchsquare status says still under review at a Nature journal.
It is moving thru peer review at glacial pace: @researchsquare status says still under review at a Nature journal.
Below is my summary of Chinese CDC preprint when it posted last year:
Basically, it reports no animal samples were SARS2 positive, but some environmental samples were.
It concludes positive environmental samples consistent w shedding by infected humans.
https://twitter.com/jbloom_lab/status/1497627179965845506
Basically, it reports no animal samples were SARS2 positive, but some environmental samples were.
It concludes positive environmental samples consistent w shedding by infected humans.
At the time it posted, I noted two caveats associated with Chinese CDC pre-print.
First caveat was that no raw data (FASTQ files) were available from sequencing of the samples:
First caveat was that no raw data (FASTQ files) were available from sequencing of the samples:
https://twitter.com/jbloom_lab/status/1497627181836500994
Second caveat is that all samples were collected on or after Jan-1-2020:
Human infections started in Wuhan no later than Nov 2019, which limits how much can be concluded from either positive or negative samples collected on Jan 1.
https://twitter.com/jbloom_lab/status/1497627183367417858
Human infections started in Wuhan no later than Nov 2019, which limits how much can be concluded from either positive or negative samples collected on Jan 1.
Chinese CDC collected samples throughout market, but focused on southwest corner where animals sold. Because sampling focused on southwest corner, both negative (green) and positive (orange) environmental samples were mostly from that part of market. 
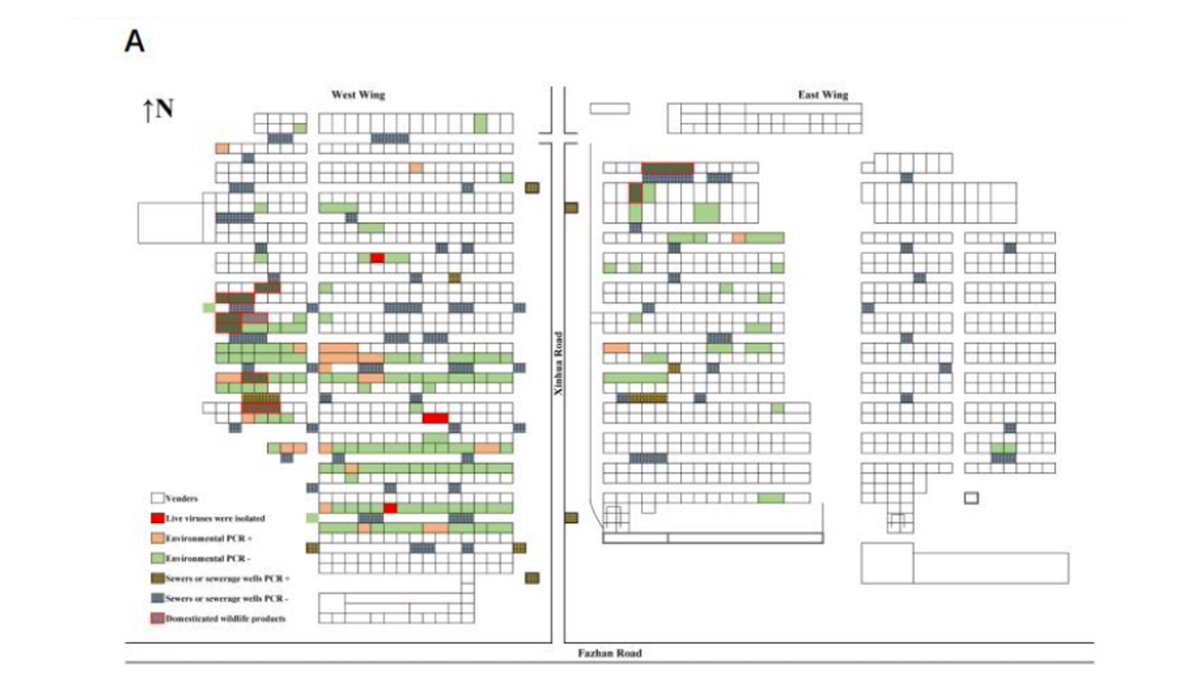
Chinese CDC reports testing samples for SARS2 by PCR & deep sequencing them. They report no tendency for the rate of PCR positive samples to be associated with any type of market vendor: similar positivity rates for stalls selling wildlife, vegetables, livestock, seafood, etc. 

Table 1 summarizes environmental sample testing.
I’ve added highlighting & raccoon dog image next to sample Q61.
This sample was collected on Jan-12 & was negative for SARS2 by PCR but positive by NGS, which presumably means it had detectable SARS2 reads in deep sequencing.
I’ve added highlighting & raccoon dog image next to sample Q61.
This sample was collected on Jan-12 & was negative for SARS2 by PCR but positive by NGS, which presumably means it had detectable SARS2 reads in deep sequencing.

Does fact Q61 was negative for SARS2 by PCR testing mean it had less viral RNA than samples w measurable Ct values? Unclear, because neither Chinese CDC pre-print nor subsequent analysis discussed below quantify fraction of deep sequencing reads that map to SARS2 for all samples.
This brings us to the second analysis by Crits-Christoph et al which recently posted on Zenodo. This pre-print analyzes deep sequencing data for some samples the authors obtained via GISAID, although those data remain unavailable to others.
zenodo.org/record/7754299…
zenodo.org/record/7754299…
The way Crits-Christoph et al obtained these data has itself become a controversy. I am not going to address that. Read the first 3 pages of Crits-Christoph report linked above & GISAID statement (gisaid.org/statements-cla…) for opposing viewpoints.
Instead, I’ll focus on the substance of the Crits-Christoph analysis. Their analysis focused on the non-SARS2 metagenomic content of the deep sequenced environmental samples.
Key results are in Fig 1 of their analysis, shown below.
Contour heatmap shows fraction of positive samples from different regions of market.
Positive samples mostly from southwest corner, since that is where Chinese CDC mostly collected samples.
Contour heatmap shows fraction of positive samples from different regions of market.
Positive samples mostly from southwest corner, since that is where Chinese CDC mostly collected samples.

So contour plot just reflects where Chinese CDC collected samples (as shown in Fig 1A of their pre-print, mentioned earlier in this thread).
In other words, contour does not show percent positivity of collected samples, but total positive samples not normalized by total samples.
In other words, contour does not show percent positivity of collected samples, but total positive samples not normalized by total samples.
The novel part of Fig 1 of Crits-Christoph et al is fraction of mammalian mtDNA reads in each analyzed sample that mapped to variety of mammalian species. These are pie chart circles.
There are samples w mtDNA from humans, pigs, sheep, cows, Siberian weasels, raccoon dogs, etc.
There are samples w mtDNA from humans, pigs, sheep, cows, Siberian weasels, raccoon dogs, etc.

The sample that has attracted the most attention is the mostly light green pie circle, for which the dominant mtDNA is from a raccoon dog. This has attracted attention because raccoon dogs are one of several species known to be susceptible to SARS2.
That green pie circle is sample Q61 from Chinese CDC pre-print, which was SARS2 negative by PCR but positive by deep sequencing. When data become available, I would like to quantify SARS2 reads in each sample, as it would be useful to know how much SARS2 was in each sample.
For instance, it is unclear whether there is enough SARS2 in the raccoon dog mtDNA dominated Q61 sample to actually infer a viral sequence, or if the undetectable Ct value for that sample means there are just trace levels of reads that are too low to obtain a viral sequence.
There are also lots of pie circles where the dominant mtDNA is from humans, & also from animals that were probably only sold as meat (eg, cow, sheep, pig). And although not shown in figure (since it’s not a mammal), the text reports some samples had fish mtDNA.
So as Crits-Christoph et al note in text, fact that mtDNA from a species is found in a sample doesn’t mean that species was infected w SARS2. It just means material from that animal ended up in the same place as viral RNA, which was widespread in the Huanan Market by Jan 2020. 

They also analyze a few samples in more detail, including assembling near-complete mtDNA for some animals, and some genomic / cDNA contigs for sample Q61. This could be useful for learning more details about animals from which genetic material in the market was derived.
Overall, main thing we learn is details of which animals or products (eg, meats) were in market before it closed on Jan-1-2020.
This doesn’t tell us if any infected w SARS2. But knowing more about animals could help trace supply chain, which is valuable line of investigation.
This doesn’t tell us if any infected w SARS2. But knowing more about animals could help trace supply chain, which is valuable line of investigation.
However, human SARS2 infections started in Wuhan no later than Nov 2019. So we have to be circumspect about environmental samples from Jan 2020.
Eg, raccoon dog sample Q61 was collected on Jan-12-2020, which is at least 6 and probably >8 weeks after first human infections.
Eg, raccoon dog sample Q61 was collected on Jan-12-2020, which is at least 6 and probably >8 weeks after first human infections.
This brings me back to main caveat I noted about Chinese CDC pre-print when it posted last year: we are unlikely to get conclusive answers about origin of an outbreak that started in Nov 2019 (or earlier) by looking at samples collected in Jan 2020:
https://twitter.com/jbloom_lab/status/1497627224094035971
Analyses of Jan 2020 samples is definitely worthwhile, because as @DrTedros rightly noted each piece of data is important for better understanding initial outbreak in Wuhan.
But to identify actual origin of outbreak, we need details from Nov or early Dec 2019.
But to identify actual origin of outbreak, we need details from Nov or early Dec 2019.
Recall that there are descriptions of confirmed cases, both unlinked and linked to the market, dating back to Nov and very beginning of Dec 2019:
https://twitter.com/jbloom_lab/status/1462231909430267906
See also this case timeline from an early paper:
If we ever learn origin of SARS2, I suspect it will come from information on cases or events that preceded those first reported cases.
https://twitter.com/michaelzlin/status/1637344158372474881
If we ever learn origin of SARS2, I suspect it will come from information on cases or events that preceded those first reported cases.
• • •
Missing some Tweet in this thread? You can try to
force a refresh