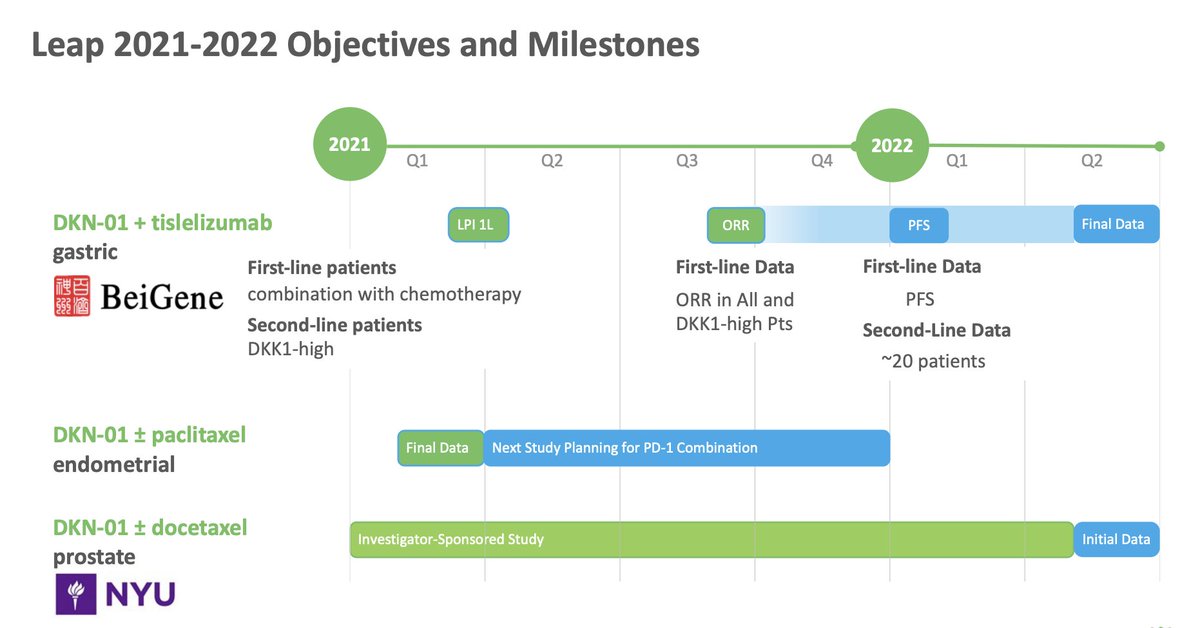
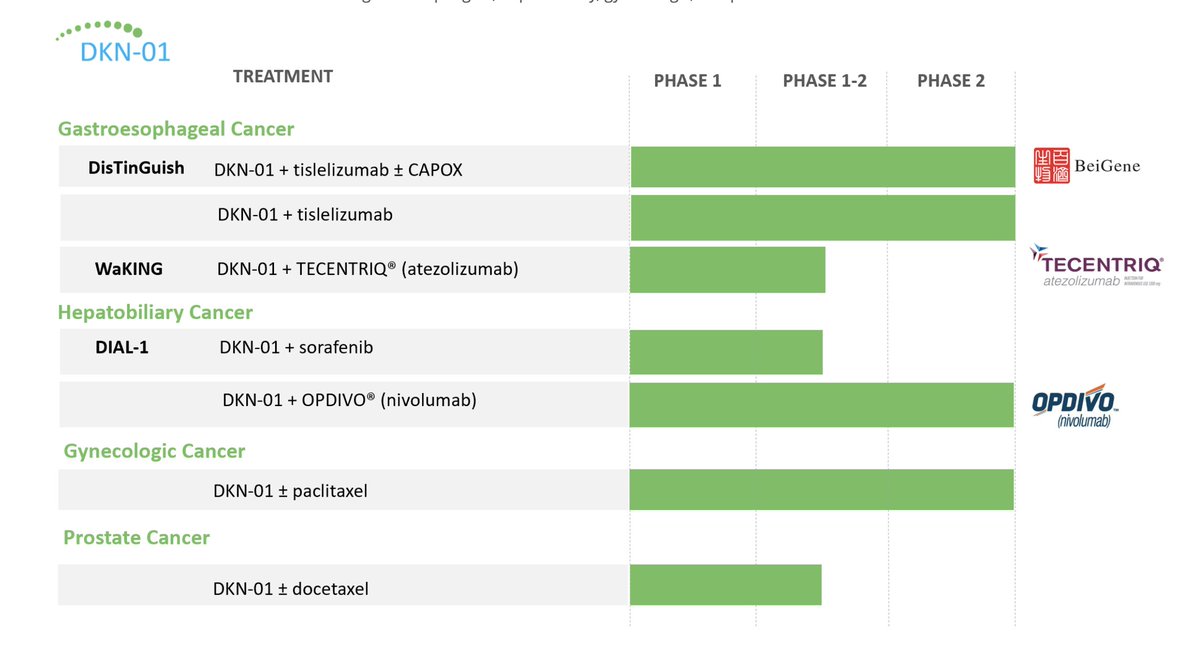
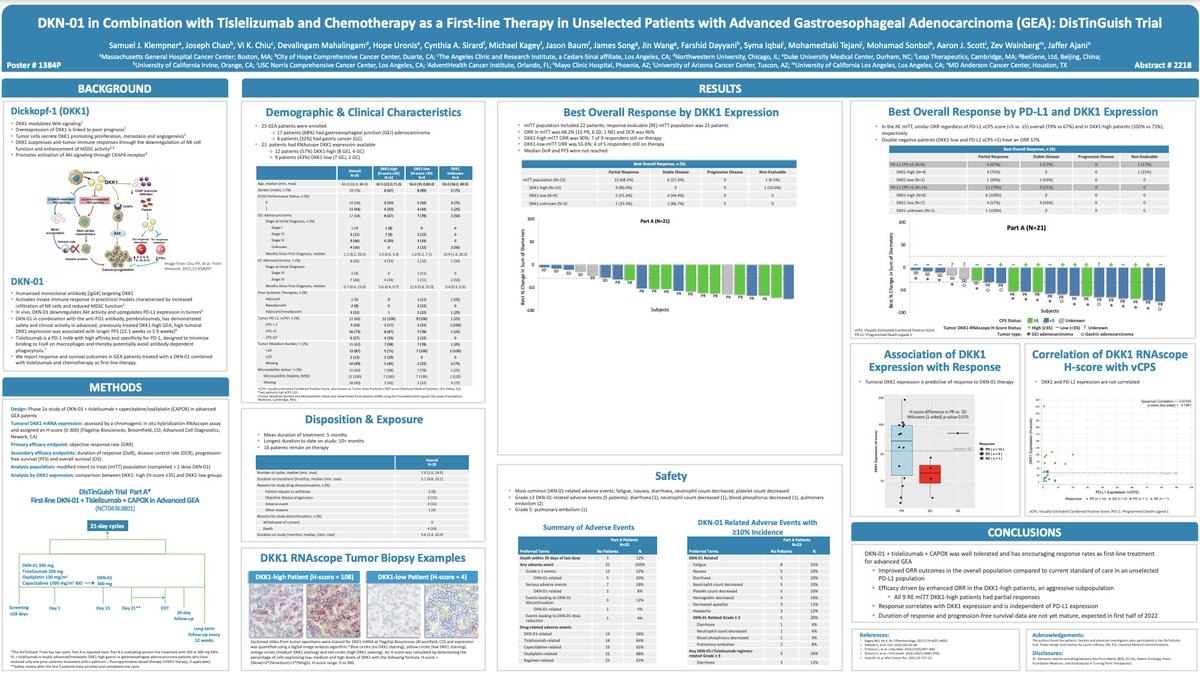
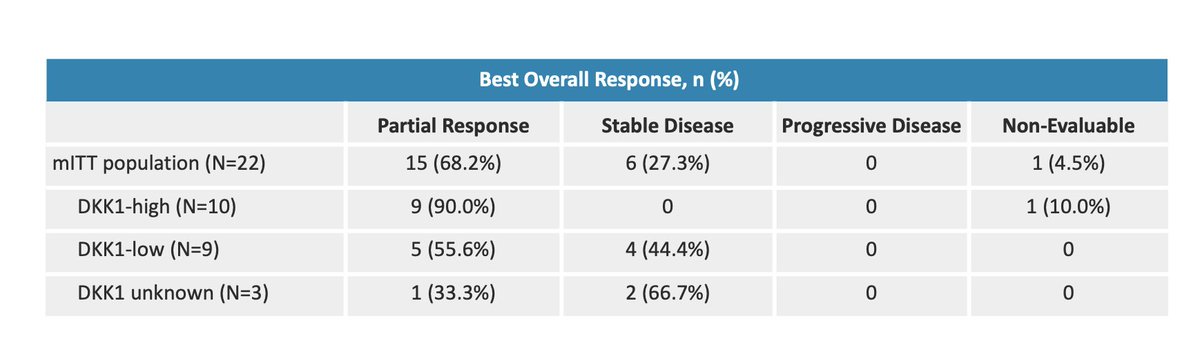
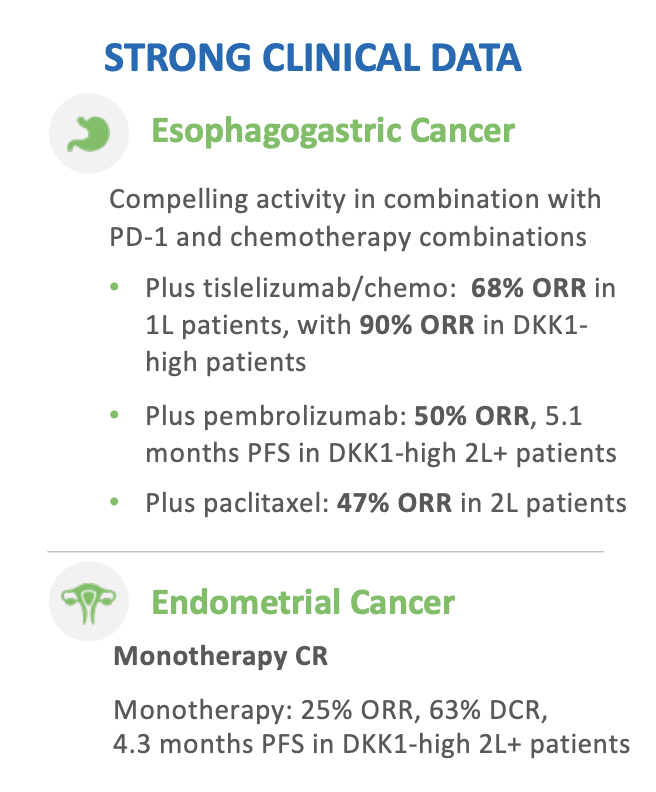
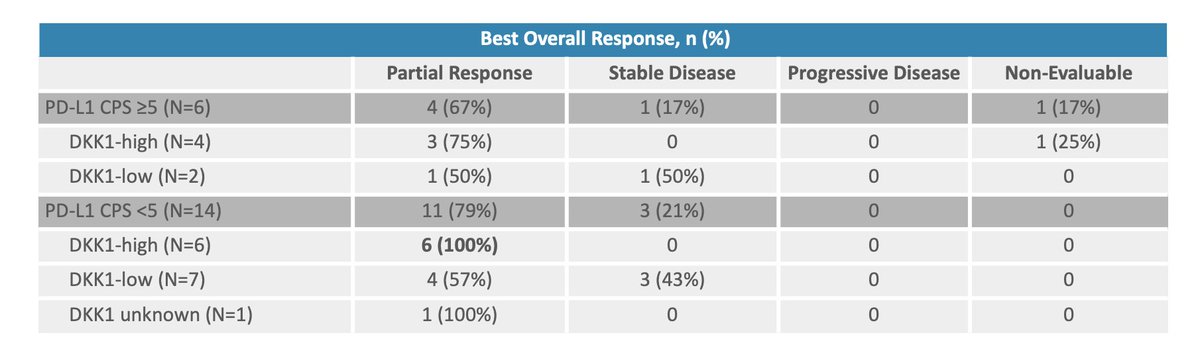
$MDXH thread here on advantages . MDxHealth is a biotechnology company that specializes in developing and commercializing molecular diagnostic tests that aid in the diagnosis and treatment of urologic cancers. Advantages include: 1/6
1. Accurate and Early Detection: $MDXH diagnostic tests can detect cancer at an early stage, even before symptoms appear. This allows for earlier intervention and treatment, which can improve patient outcomes. 2/6
2. Personalized Treatment: $MDXH MDxHealth's tests provide information about the specific characteristics of a patient's tumor, allowing doctors to personalize treatment plans and select the most effective therapies. 3/6
3. Non-invasive: MDxHealth's diagnostic tests are non-invasive and require only a urine or blood sample, making them less painful and less invasive than traditional diagnostic methods. 4. Cost-effective: $MDXH tests are cost-effective, as they can reduce the need for more 4/6
expensive diagnostic procedures and improve patient outcomes, ultimately reducing healthcare costs. 5. Innovation: MDxHealth is constantly innovating & developing new diagnostic tests, which can improve the accuracy and effectiveness of cancer detection and treatment. 5/6 $MDXH
Overall, MDxHealth's molecular diagnostic tests offer a number of advantages for both patients and healthcare providers, including earlier detection, personalized treatment, non-invasive testing, cost-effectiveness, and ongoing innovation. $MDXH
• • •
Missing some Tweet in this thread? You can try to
force a refresh