#ECCMID2023 1/3. Alex Soriano: PO the new IV? Need to consider oral bioavailability, pathogen MICs, delays to Cmax, inter-individual variability in BA etc 



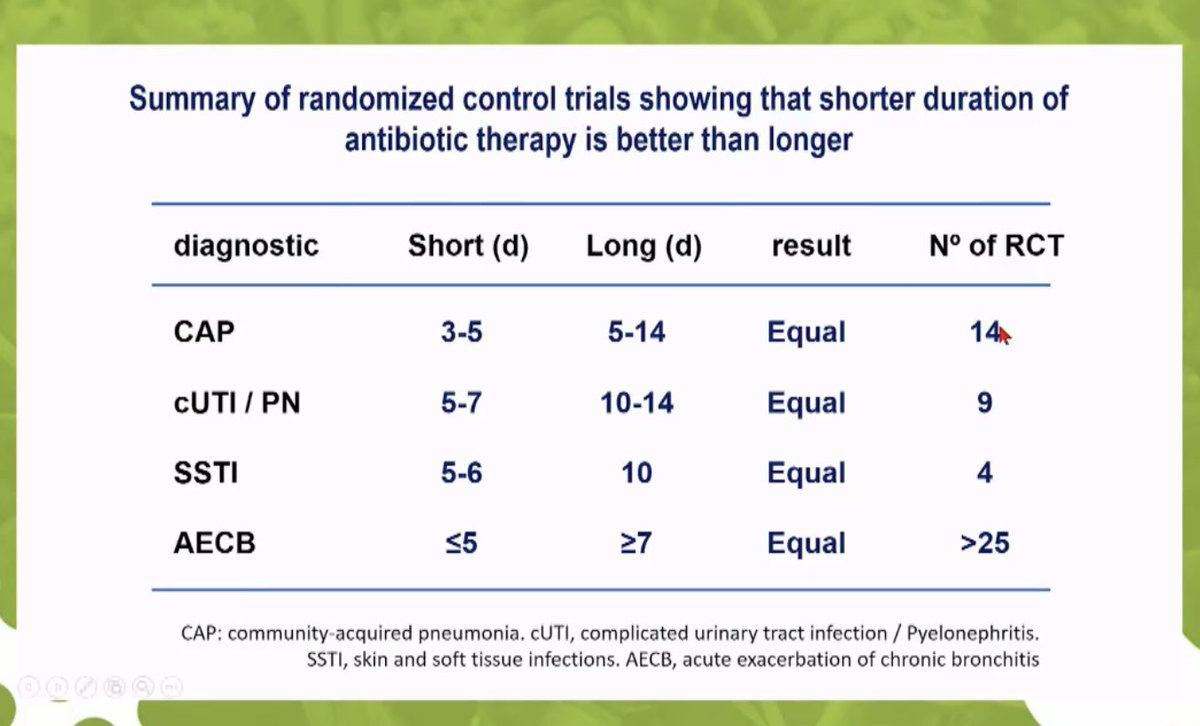
2/3 Numerous RCTs now demonstrate short course therapy is adequate for most syndromes; RCT of oral switch in severe CAP is safe and reduced LoS pubmed.ncbi.nlm.nih.gov/17090560/ 
3/3 Early oral switch in non-critically ill febrile patients: The acute phase of infection does not influence the exposure or PTA for PO amoxicillin or cipro
pubmed.ncbi.nlm.nih.gov/36433818/
pubmed.ncbi.nlm.nih.gov/36433818/
Another important point - effect of oedema in soft tissue infection, increasing the volume of distribution which may compromise lower oral dosing 

• • •
Missing some Tweet in this thread? You can try to
force a refresh
 Read on Twitter
Read on Twitter








