We’ve identified the somato-cognitive action network (SCAN), a newly recognized network within human primary motor cortex that disrupts the famous—but incorrect—motor homunculus, and has strong connections to high-level control networks. Now out in @Nature nature.com/articles/s4158… 
We used data from single individuals, large-N group averages, children, infants, neonates, stroke patients, and macaques, including resting fMRI, task fMRI, diffusion, and structure, to map primary motor cortex in exquisite detail. We were surprised at what we found!
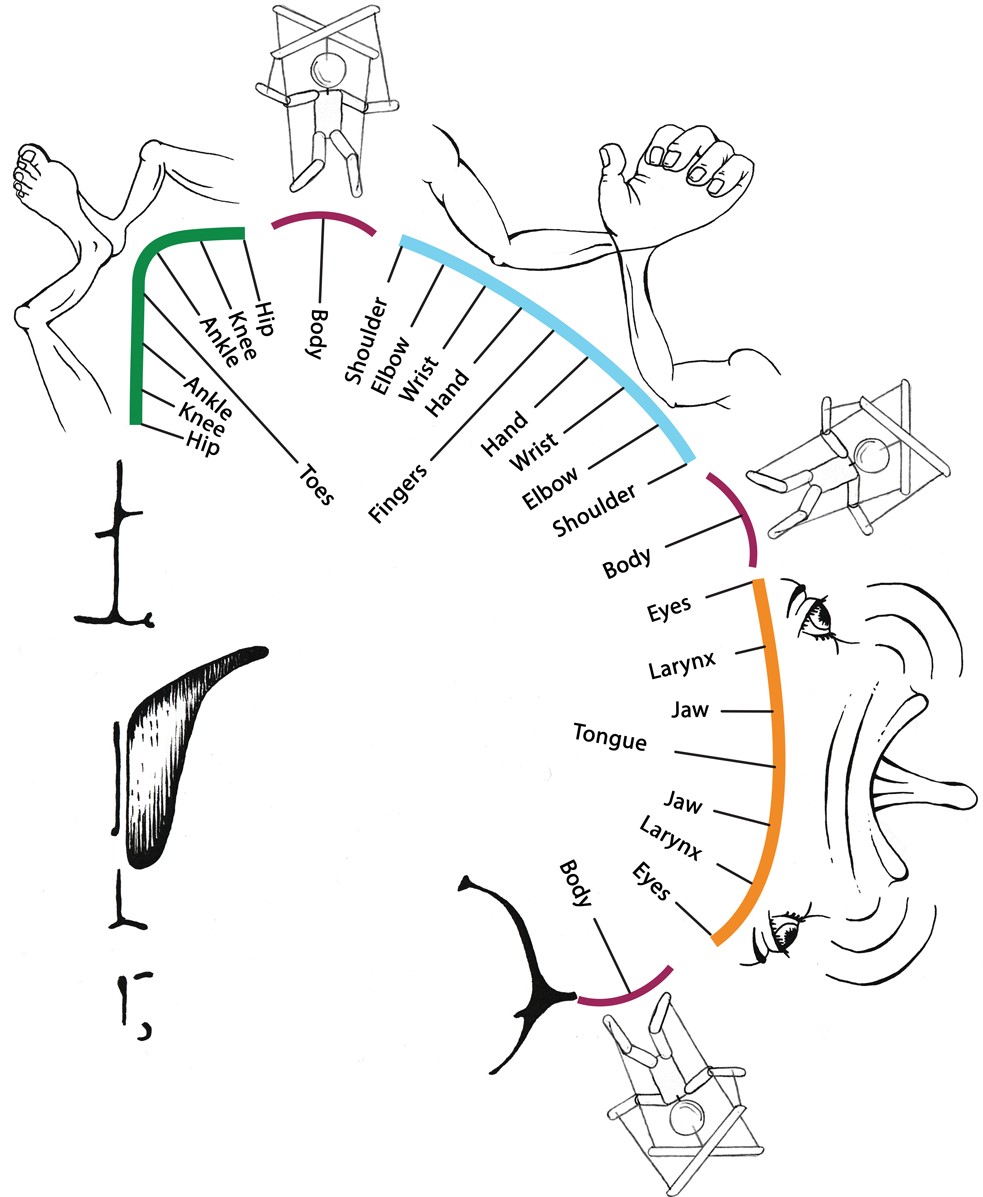
First, some context. One foundational principle of brain organization we all learn in introductory neuroscience class is Wilder Penfield’s motor homunculus, which describes a linear progression of motor representation in precentral gyrus running from feet to face. 

Noninvasive FMRI examinations of M1 organization—both task and resting-state, including our own—previously showed the presence of at least separable foot/hand/face divisions. This is basically consistent with Penfield’s homunculus. 



We aimed to describe the homunculus in more detail using Precision Functional Mapping (PFM), where we collect large amounts of high-quality resting-state fMRI and task data from single individuals.
Reader, we did not find a homunculus.
Reader, we did not find a homunculus.
Resting-state connectivity in single individuals from seeds along precentral gyrus showed three regions corresponding to the effector-specific foot, hand, and face regions of M1, corresponding with the homunculus model and matching prior work exactly. 

But we also found three OTHER regions interleaved between the effector-specific regions. These “inter-effector” areas were strongly functionally connected to each other. They formed a distributed functional network within primary motor cortex (!!!). 

The existence of this inter-effector system surprised us. A set of distributed, connected regions within M1? This is a dramatic violation of the homunculus! We had to validate it in other data.
We found it in each of our PFM subjects, and replicated it within-subject.
We found it in each of our PFM subjects, and replicated it within-subject.

Maybe this is something strange about our MRI sequence or processing? We looked at advanced multi-echo data sent to us by @cl9681, @immanuel_elbau, Jonathan Power, and Conor Liston. It was clear in those individuals as well (one subject also showed strong FC to the mouth area). 

If it’s in EVERY subject, why hasn’t it been noticed in the big publicly available group-average datasets before?
Well, has anyone ever looked for it?
Let’s look now.
Well, has anyone ever looked for it?
Let’s look now.
We found the same inter-effector network in UK Biobank data (n=4000).
And in ABCD data (n=3900).
And in Human Connectome Project data (n=812).
And in our old WashU data (n=120).
And in ABCD data (n=3900).
And in Human Connectome Project data (n=812).
And in our old WashU data (n=120).

The inter-effector network was more difficult to find in the group-average datasets. Boundaries between regions were less sharp, and we had to turn up the visualization threshold to see the network come out. But it was there. 

In all of these individual and group datasets, these two types of connectivity patterns—foot/hand/mouth effector-specific and distributed inter-effector—were the only patterns observed.
So this novel inter-effector network is present in every individual adult examined. Well, what about children? We partnered with @DeannaJGreene, @ChadMSylvester, John Pruett, and Chris Smyser to examine PFM data from pediatric populations.
We found the same inter-effector network in data from a nine-year-old.
And from an 11-month old (!).
But NOT from a neonate (scanned beginning at 13 days old).
Its absence was verified in group average data from a large set of neonates (n=262).



And from an 11-month old (!).
But NOT from a neonate (scanned beginning at 13 days old).
Its absence was verified in group average data from a large set of neonates (n=262).




So we have observed a distributed brain network within primary motor cortex that violates the homunculus and emerges within the first year of life. But what does it do? First, we described its connectivity.
Outside of M1, the inter-effector regions are most strongly connected to regions in medial prefrontal cortex (SMA, dACC), dorsal posterior putamen, centromedian thalamus, and cerebellum (anterior lobule and vermis). 

Ok, but what we really want to know is how the inter-effector connectivity differs from connectivity of the effector regions.
It turns out that the inter-effectors are much more strongly connected to the individual-specific Cingulo-Opercular Network (CON).

It turns out that the inter-effectors are much more strongly connected to the individual-specific Cingulo-Opercular Network (CON).

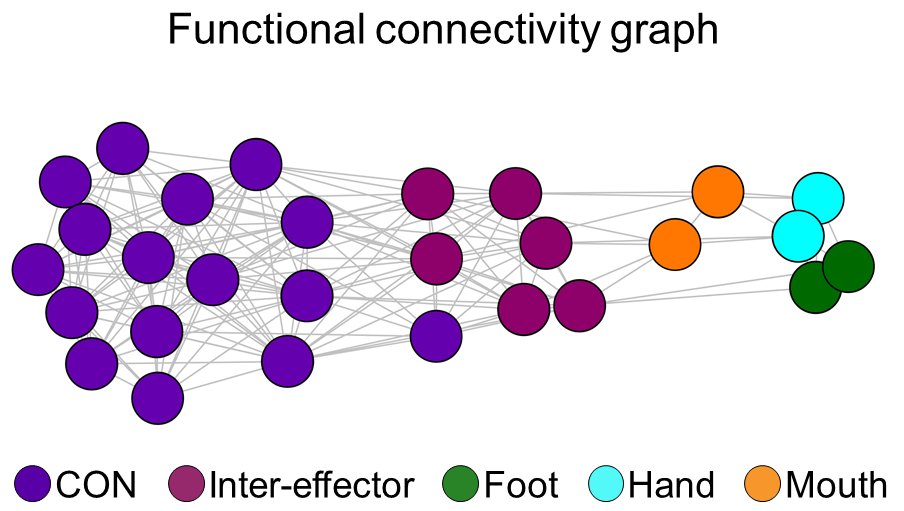
This is wild—CON is a high-level network for executive function and top-down control. It sets and maintains goal-relevant action plans. And it’s connected directly to this inter-effector network in M1?
Yes, it is, in every subject.

Yes, it is, in every subject.


How else does the inter-effector network differ from the effector-specific areas? Well, it has stronger connectivity with the middle insula, the cerebellar vermis, and the posterior dorsal putamen. 

It also has stronger connectivity with a number of thalamic nuclei, including the centomedian, ventral intermediate, and ventral posteriomedial. But it has weaker connectivity with adjacent S1. 

Structurally, we found that the inter-effector areas were thinner than the effector-specific areas. Very interesting—M1 is overall one of the thickest parts of cortex, but here a portion of M1 is relatively thin—more like prefrontal cortex. 

We had diffusion data in three subjects. Fractional anisotropy—a measure of white matter coherence—was much higher directly under the inter-effector network than under the effector-specific regions. 

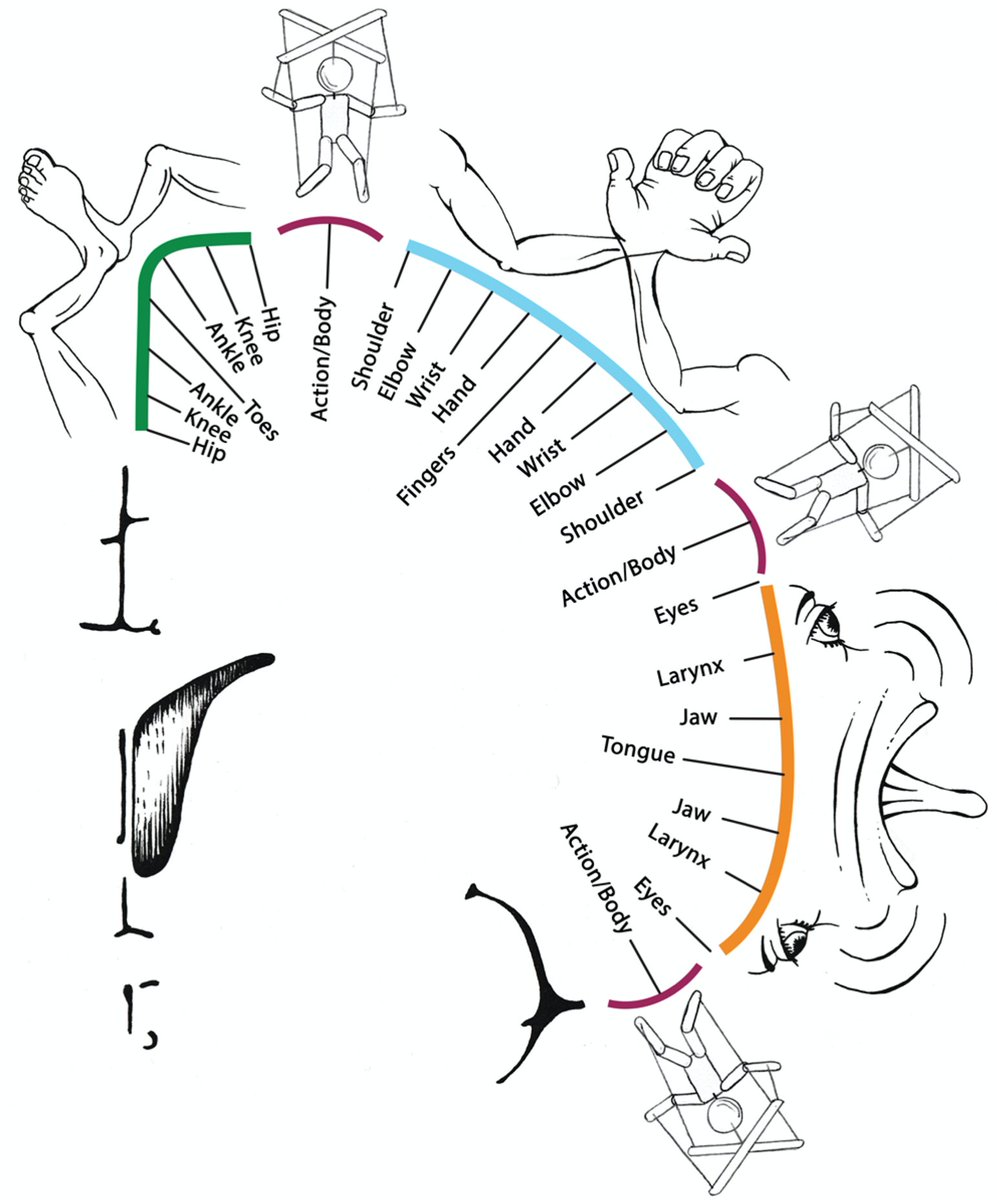
How do the inter-effectors fit into the homunculus? We scanned an extensive series of movement tasks to map movement representation. Again, findings did not follow a homuncular model. Preferred movements followed a *concentric* organization, not a linear toes-to-face one. 

Specifically, within each of the foot/hand/mouth regions, the most distal body parts (e.g., hand) were represented in the center of the region, surrounded by concentric rings of more proximal body parts (wrist-elbow-shoulder). 

The inter-effector regions were at the intersection of these foot / hand / mouth concentric rings, and were nonspecifically coactivated during many movements (not what you would expect from a homuncular organization). 

Proximal body parts (abdominals, eyebrow, swallowing) were most strongly represented in inter-effector regions, but representation was distributed—when they activated, all three regions coactivated, plus CON. 

We then had subjects perform an action control task in which execution of complex movements was preceded by a planning phase. Inter-effector regions were more activated during planning than execution. 

Wait, hold on. We’ve been mapping M1 using direct cortical stimulation and recording since the ‘30s. How has no one has noticed this network?
Well, if stimulating inter-effectors doesn’t produce obvious, isolated movements (>50% of stimulations don’t), it wouldn’t be noticed.
Well, if stimulating inter-effectors doesn’t produce obvious, isolated movements (>50% of stimulations don’t), it wouldn’t be noticed.
Roux et al. (doi.org/10.1113/JP2801…) reported an M1 region where stimulation never produced movement across N=100 patients. We mapped their successful stimulations to the cortical surface. They cleanly avoided the middle inter-effector. 



And since our initial preprint, Jensen et al. posted a preprint (biorxiv.org/content/10.110…) where they recorded directly from M1 locations convergent with our inter-effector network. These regions nonspecifically coactivated during motion of multiple different effectors. 

Wait, hold on. M1 has been mapped for decades in macaques. Do macaques have a network between the foot/hand/face areas?
We investigated macaque fMRI data collected by @DrDamienFair @_janzimmermann_ and by the PRIME-DE consortium (thanks @TingsterX @benediktramirez).
We investigated macaque fMRI data collected by @DrDamienFair @_janzimmermann_ and by the PRIME-DE consortium (thanks @TingsterX @benediktramirez).
A seed placed in macaque caudal cingulate cortex—but not rostral cingulate cortex—revealed distributed regions of strong functional connectivity in M1 between the mouth/hand and hand/foot areas, in individual and group average macaque data. 

This FC pattern—medial PFC plus two M1 regions between foot/hand/face—corresponds very well with regions the great Peter Strick has shown to project to internal organs such as stomach and adrenal medulla (pnas.org/doi/abs/10.107…) 

Together, this work describes an integrated M1 system distinct from the rest of M1. While the foot/hand/face areas are optimized for isolated movements critical for e.g. speaking or writing, this novel system more likely enables integrated, whole-body movement.
Notably, this system also plans actions, maybe regulates physiology via internal organ projections, and connects to a control network. This suggests a system that enables allostatic, *anticipatory* body regulation.
Think about it: when you are going to do something dangerous or stressful, when do you notice sweating/body tension/heart rate elevation/butterflies in your stomach? Not when you start the activity, but when you plan it. This prepares you in advance for difficult situations.
The CON likely transmits action plans to this M1 system to initiate such somatic/physiological effects. That is, the system serves as an interface between your somatic body and your cognitive systems that plan and control actions. A somato-cognitive action network (SCAN)!
The integrated SCAN sits at the edge of three concentric functional zones in M1 that allow precise isolated control of individual effectors.
We propose this dual-system, integrate-isolate model of M1 as an update to Penfield’s famous linear homunculus.
We propose this dual-system, integrate-isolate model of M1 as an update to Penfield’s famous linear homunculus.

There are more findings in the paper (yes, even more) that I don’t have space for here (Differences between SCAN regions! Stroke patients! Dual laryngeal representations!). I encourage everyone who’s interested to dive in. nature.com/articles/s4158… 

I have to say that “revising the motor homunculus” was not the plan when I started mapping M1 connectivity, but sometimes you have to go where the data leads you. I think it has taken us somewhere amazing!
Thank you to all who contributed data, effort, & brainpower. Credit to @RoselyneChauvin for motor tasks; @vannatandrew @TingsterX for macaque work; and to @DrDamienFair, Tim Laumann, Marc Raichle, Mike Graziano, and Peter Strick for helping shape our ideas about this network.
Finally, data from this study came from many many collaborators, much of it now publicly available.
P1-3: openneuro.org/datasets/ds002…
Stroke patient: openneuro.org/datasets/ds004…
Thanks to Noah Baden for the huge effort of getting our data ready to be publicly shared!
P1-3: openneuro.org/datasets/ds002…
Stroke patient: openneuro.org/datasets/ds004…
Thanks to Noah Baden for the huge effort of getting our data ready to be publicly shared!
UKB: fmrib.ox.ac.uk/ukbiobank/
ABCD: doi.org/10.15154/15032…
HCP: humanconnectome.org
Wash U: openneuro.org/datasets/ds000…
PRIME-DE: fcon_1000.projects.nitrc.org/indi/PRIME/oxf…
ABCD: doi.org/10.15154/15032…
HCP: humanconnectome.org
Wash U: openneuro.org/datasets/ds000…
PRIME-DE: fcon_1000.projects.nitrc.org/indi/PRIME/oxf…
• • •
Missing some Tweet in this thread? You can try to
force a refresh