Buckle up for the 1st installment of our HF #Tweetorial series w/ help from @AHajduczok on HF #Hemodynamics based on the @JCardFail State of the Art Review by @stevenhsu_md, @JamesCFangMD and #barryborlaug. An absolute must read for ALL (not only HF folks) 🔥
@robmentz @dranulala
@robmentz @dranulala
First, check out this grade A #tweetorial by @AHajduczok who helps de-mystify Pressure Volume (PV) Loops:
(more to come SOON on this Part III: devices and drugs in PV loops cc @RyanTedfordMD @PSullivan000 @BurkhoffMd)
(more to come SOON on this Part III: devices and drugs in PV loops cc @RyanTedfordMD @PSullivan000 @BurkhoffMd)
https://twitter.com/AHajduczok/status/1583429179915132935
The Normal LV volume loop relationships illustrate:
⚠️End-Systolic Pressure Volume Relationship (ESPVR)➡️Ventricular Contractility
⚠️End Diastolic Pressure Volume Relationship (EDPVR)➡️Diastolic Compliance
⚠️EDV-ESV=Stroke Volume (SV)
⚠️End-Systolic Pressure Volume Relationship (ESPVR)➡️Ventricular Contractility
⚠️End Diastolic Pressure Volume Relationship (EDPVR)➡️Diastolic Compliance
⚠️EDV-ESV=Stroke Volume (SV)

PV Relationship in patients with HFrEF
Decreased Contractility (ESPVR)
Increased End Diastolic Volume
Near-Normal SV
⚠️Reduced EF is because of increased end diastolic volume, not decreased Stroke Volume
Decreased Contractility (ESPVR)
Increased End Diastolic Volume
Near-Normal SV
⚠️Reduced EF is because of increased end diastolic volume, not decreased Stroke Volume

So what here represents afterload?
Afterload is the effective arterial elastance (Ea)
⚠️Ea reflects the STIFFNESS of the vasculature
Ea = End Systolic Pressure(ESP)/SV
–directly proportional to SVR and HR
–inversely proportional to arterial compliance
Afterload is the effective arterial elastance (Ea)
⚠️Ea reflects the STIFFNESS of the vasculature
Ea = End Systolic Pressure(ESP)/SV
–directly proportional to SVR and HR
–inversely proportional to arterial compliance
What about afterload in HFrEF?
‼️Shallow ESPVR in chronic HFrEF results in increased afterload-sensitivity (from LV remodeling)
✅Diuresis in HFrEF results in ⬇️ LVEDV, ⬇️ Ea, leading to marked ⬆️ in SV––Improving congestive symptoms!
‼️Shallow ESPVR in chronic HFrEF results in increased afterload-sensitivity (from LV remodeling)
✅Diuresis in HFrEF results in ⬇️ LVEDV, ⬇️ Ea, leading to marked ⬆️ in SV––Improving congestive symptoms!

So how do inotropes and MCS affect the hemodynamics in heart failure?
Lets start with Inotropes:
Increase ⬆️ ESPVR and ⬇️ systemic vascular resistance
Net Effects:
⬆️SV
⬇️Decreased Afterload
⬇️Decreased end diastolic pressure
What's the cost?
--Increased myocardial O2 demand
Lets start with Inotropes:
Increase ⬆️ ESPVR and ⬇️ systemic vascular resistance
Net Effects:
⬆️SV
⬇️Decreased Afterload
⬇️Decreased end diastolic pressure
What's the cost?
--Increased myocardial O2 demand

MCS effects can be confusing; but let's break down the devices:
IABP counterpulsation indirectly augments flow from LV to systemic circulation by:
Decreasing Ea
Increasing SV while decreasing end diastolic volume and pressure
⚠️This only works for an LV with sufficient ESPVR⚠️
IABP counterpulsation indirectly augments flow from LV to systemic circulation by:
Decreasing Ea
Increasing SV while decreasing end diastolic volume and pressure
⚠️This only works for an LV with sufficient ESPVR⚠️

Percutaneous microaxial flow pumps actively draw blood from LV into the aorta resulting in:
–Decreased LV Filling pressures and Myocardial demand
–Increased Systemic outflow and Mean Arterial Pressure
The triangular PV loop ➡️ continuous LV emptying (no Ao valve closing corner)
–Decreased LV Filling pressures and Myocardial demand
–Increased Systemic outflow and Mean Arterial Pressure
The triangular PV loop ➡️ continuous LV emptying (no Ao valve closing corner)

Peripheral VA-ECMO: To Vent or Not to Vent that is the question!
Why consider venting? Peripheral VA-ECMO results in a significant afterload against a weak LV
Venting (impella):
–Decreased Afterload results in decreased EDP preventing fluid overloaded states and improving SV
Why consider venting? Peripheral VA-ECMO results in a significant afterload against a weak LV
Venting (impella):
–Decreased Afterload results in decreased EDP preventing fluid overloaded states and improving SV

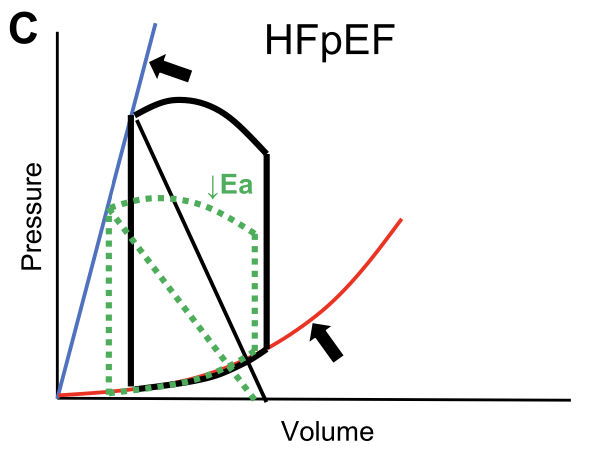
What about HFpEF?
In HFpEF there is commonly an EDPVR shift up and to the left, owing to increases in viscoelastic chamber stiffness.
This results in higher filling pressures relative to chamber volume (bottom arrow), an effect that is amplified at higher volumes...
In HFpEF there is commonly an EDPVR shift up and to the left, owing to increases in viscoelastic chamber stiffness.
This results in higher filling pressures relative to chamber volume (bottom arrow), an effect that is amplified at higher volumes...

In addition to active stiffening (ie, contractility), the ESPVR is increased through passive chamber stiffening, such that the ESPVR is increased in HFpEF, even as other measures of chamber and myocardial contractility are depressed (upper arrow). 

Increased systolic and diastolic stiffness, increases sensitivity to diuresis and vasodilators.
Reducing afterload results ⬇️ BP without significant ⬆️ in SV
The increase in the EDPVR renders them more sensitive to LV preload reduction with venodilators, which decreases the SV
Reducing afterload results ⬇️ BP without significant ⬆️ in SV
The increase in the EDPVR renders them more sensitive to LV preload reduction with venodilators, which decreases the SV
Enough about the LV, let's discuss RV Failure (REQUIRED READING: nejm.org/doi/full/10.10… @Brian_Houston12 @RyanTedfordMD)
The RV PV loop differs in the amplitude of pressure change and takes on a more trapezoidal/triangular shape
‼️Accepts the same CO at 1/5th the pressure‼️
The RV PV loop differs in the amplitude of pressure change and takes on a more trapezoidal/triangular shape
‼️Accepts the same CO at 1/5th the pressure‼️

Contractility (Ees) should match the Ea for a Ees/Ea ratio ≥1. But in Pulmonary Hypertension:
–Ea increases as the PA pressure increases
If the RV is compensated, the Ees also increases maintaing a ratio of ≥1 and RV EF
–Ea increases as the PA pressure increases
If the RV is compensated, the Ees also increases maintaing a ratio of ≥1 and RV EF

What about when the RV is decompensated?
–Afterload (Ea) Increases
–Contractility (Ees) decreases
Resulting in RV dilation to maintain SV, but similarly to HFrEF in LV:
⚠️EDV increases resulting in decreased EF
–Afterload (Ea) Increases
–Contractility (Ees) decreases
Resulting in RV dilation to maintain SV, but similarly to HFrEF in LV:
⚠️EDV increases resulting in decreased EF

Progressive decompensation of RV results in:
–Worsening RV contractility and diastolic compliance
–Decrease in RV Ees/Ea ratio,
–Decrease in CO
–increase in RV EDP and right atrial pressure (RAP) out of proportion to left-sided pressure.
–Worsening RV contractility and diastolic compliance
–Decrease in RV Ees/Ea ratio,
–Decrease in CO
–increase in RV EDP and right atrial pressure (RAP) out of proportion to left-sided pressure.
‼️RV compensation may mask RV Disease thus stressing the RV may reveal pathology unapparent at rest
What about the Pericardiums role in HF and Pulmonary Hypertension?
The RV and LV are connected in series because the RV provides flow to the RV right? @JACCJournals
sciencedirect.com/science/articl….
The RV and LV are connected in series because the RV provides flow to the RV right? @JACCJournals
sciencedirect.com/science/articl….

But they also have a parallel relationship through the phenomenon of ventricular interaction:
The restraining effects of the pericardium amplify competition between the right and left heart for volume!
The restraining effects of the pericardium amplify competition between the right and left heart for volume!
Chamber pressure measured with a catheter represents the sum of transmural filling pressure (the forces from within favoring distention) together with the external pressure applied on the epicardium that, when positive, opposes distention.
‼️Pericardial Pressure=RA pressure‼️
‼️Pericardial Pressure=RA pressure‼️

In normal cardiac conditions, due to thicker walls, the LV has a steeper EDPVR than the RV.
⚠️Pericardial restraint on the heart is minimal
⚠️Pericardial restraint on the heart is minimal

However, acute increases in cardiac volume, due to exercise, pulmonary embolism, or acute valve insufficiency, increases in pericardial restraint are augmented.
Resulting in: ⬇️
Resulting in: ⬇️
Increases in surface contact pressure on the epicardium transmit to increase intracavitary pressure
Thus the intracavitary pressure is increased even if the transmural distending pressure is normal or even low, as often noted during acute decompensated HF as described below
Thus the intracavitary pressure is increased even if the transmural distending pressure is normal or even low, as often noted during acute decompensated HF as described below
RV distention due to central venous congestion shifts the LV EDPVR leftward due to increased pericardial restraint thus
–Decreasing LV transmural filling pressure
–Decreasing LV Preload
–Decreasing CO
–Decreasing LV transmural filling pressure
–Decreasing LV Preload
–Decreasing CO

⚠️Diuresis and venodilation in acute decompensated HF results in:
–Restoring LV transmural filling pressure
–Raising the LVEDV aka preload
Thus improving CO
–Restoring LV transmural filling pressure
–Raising the LVEDV aka preload
Thus improving CO
Resting hemodynamic during early HF may be normal and HF may only become evident during the stressed state, when the heart fails to respond to the heightened physiologic demands of exercise.
Fick principle: oxygen consumption (VO2) is equal to the product of CO and the arteriovenous oxygen difference (AVO2-diff).
Mathematically:
VO2=CO(AVO2-diff)
Mathematically:
VO2=CO(AVO2-diff)
Physical exertion results in an increase in VO2 that is achievable through a combined increase in CO and increase in AVO2-diff.
Thus during exercise there is an:
–Increase in oxygen delivery
–Enhanced distribution and extraction of oxygen in skeletal muscle
Thus during exercise there is an:
–Increase in oxygen delivery
–Enhanced distribution and extraction of oxygen in skeletal muscle
So how does CO increase at a rate of 6mL/min for every 1mL/min increase in VO2?
⚠️By Increasing HR and SV
SV increases due to:
–Increased venous return augmenting preload
–Increased contractility
–Decreased vascular resistance in the pulmonary and systemic circulation
⚠️By Increasing HR and SV
SV increases due to:
–Increased venous return augmenting preload
–Increased contractility
–Decreased vascular resistance in the pulmonary and systemic circulation
The increase in venous return during exercise is related to the combined actions of the skeletal muscle pumps + venoconstriction in the large capacitance veins of the abdomen.
➡️increasing the stressed blood volume (the volume that contributes to increasing vascular pressure)
➡️increasing the stressed blood volume (the volume that contributes to increasing vascular pressure)
Venous return must equal the CO and during exercise this is achieved through biventricular lusitropic reserve:
‼️Greater ventricular filling volumes despite a shorter diastolic time interval‼️
‼️Greater ventricular filling volumes despite a shorter diastolic time interval‼️
Biventricular lusitropic reserve during exercise:
➡️Results in a minimal increase in left atrial pressure and RAP
Thus:
➡️Maintaining normal pulmonary capillary and PA hydrostatic pressures
➡️Results in a minimal increase in left atrial pressure and RAP
Thus:
➡️Maintaining normal pulmonary capillary and PA hydrostatic pressures
In HF, these adaptations are limited due to:
–Increased arterial stiffness
–Inadequate vascular vasodilation
Both leading to:
⚠️Increased LV afterload and PCWP increase
–Increased arterial stiffness
–Inadequate vascular vasodilation
Both leading to:
⚠️Increased LV afterload and PCWP increase
PCWP is further increased by:
–Impaired LV and RV systolic reserve limiting SV
–Impaired diastolic relaxation and compliance
–Impaired LV and RV systolic reserve limiting SV
–Impaired diastolic relaxation and compliance
What happens with theres a profound increase in afterload and PCWP during exersion in patients with HF?
‼️Increased fluid filtration across the pulmonary capillary-alveolar interface resulting in extravascular lung water‼️
‼️Increased fluid filtration across the pulmonary capillary-alveolar interface resulting in extravascular lung water‼️

Chronotropic incompetence limits CO reserve and depression in peak VO2 is related to a blunted agumentation of AVO2-diff further contributing to the hemodynamic derangements in HF
So how do we measure hemodynamics accurately? Right Heart Caths (RHC); Key Points about RHC below:
✅Pressure is measured at end-expiration
✅CO is most accurately measured via Fick method
✅Oximetry identifies shunt physiology
✅PCWP is an estimate of Left atrial pressure
✅Pressure is measured at end-expiration
✅CO is most accurately measured via Fick method
✅Oximetry identifies shunt physiology
✅PCWP is an estimate of Left atrial pressure

For more best practice guidelines - you don't want to miss this gem by @MichaelCViray1 @RyanTedfordMD (don't forget to check a wedge sat cc @DavidLBrownMD)
meridian.allenpress.com/aph/article/19…
meridian.allenpress.com/aph/article/19…

During a RHC, at pressure measurements at end-expiration:
–For a spontaneously breathing patients are measure near maximum value➡️ avoid the impact of (➖) pleural pressures
–For vented patients measured at the minimum value ➡️account for the impact of (➕) pleural pressures
–For a spontaneously breathing patients are measure near maximum value➡️ avoid the impact of (➖) pleural pressures
–For vented patients measured at the minimum value ➡️account for the impact of (➕) pleural pressures
Direct Fick Method to measure CO:
CO = VO2/AVO2-diff
–AVO2-diff is the difference between arterial and mixed venous oxygen content
CO = VO2/AVO2-diff
–AVO2-diff is the difference between arterial and mixed venous oxygen content
Shunt physiology on RHC is done by identifying a step-ip in oxyhemoglobin saturation ≥8% between the SVC and PA
To localize the shunt oxygen saturation sampling is necessary from the SVC, IVC, RA, RV PA and PA Wedge
To localize the shunt oxygen saturation sampling is necessary from the SVC, IVC, RA, RV PA and PA Wedge
Shunt severity is measured by calculating the ratio of pulmonary-to-systemic flow (Qp/Qs)
(Qp/Qs) = (SAO2-Mixed Venous O2)/(PCW O2 - PA O2)
*Mixed Venous O2 taken BEFORE Step-Up*
‼️Normal Qp/Qs is 1:1‼️
–Qp/Qs > 1:1 indicates a left to right shunt;
–Qp/Qs ≥ 1.5 is significant
(Qp/Qs) = (SAO2-Mixed Venous O2)/(PCW O2 - PA O2)
*Mixed Venous O2 taken BEFORE Step-Up*
‼️Normal Qp/Qs is 1:1‼️
–Qp/Qs > 1:1 indicates a left to right shunt;
–Qp/Qs ≥ 1.5 is significant
Hemodynamic waveforms reveal key findings associated with disease
🔺Giant V-waves on PCW tracing represe decreased LA compliance
🔺Prominent Y-descents and Kussmaul's sign indicates poor RV compliance in PH
🔺Dip and plateau sign during diastole indicates severe RV dysfunction

🔺Giant V-waves on PCW tracing represe decreased LA compliance
🔺Prominent Y-descents and Kussmaul's sign indicates poor RV compliance in PH
🔺Dip and plateau sign during diastole indicates severe RV dysfunction


Coupled with a RHC, invasive hemodynamic exercise testing is the gold standard in differentiating the causes of exertional dyspnea.
Protocol should include:
1. Escalating exercise protocol
2. RAP/PA/PCWP values
3. CO measurement
4. Metabolic gas exchange
5. Blood gas sampling
Protocol should include:
1. Escalating exercise protocol
2. RAP/PA/PCWP values
3. CO measurement
4. Metabolic gas exchange
5. Blood gas sampling

The HFSA guidelines recommend against routine invasive hemodynamics in HF which has been supported by the ESCAPE Trial which demonstrated:
⚠️No Mortality benefit to routine PA catheter use in acute decompensated HF⚠️
⚠️No Mortality benefit to routine PA catheter use in acute decompensated HF⚠️
However, when volume or perfusion status is unclear hemodynamic assessment can provide actionable data
‼️The table below shows how hemodynamics can alter management and assist with therapy in common clinical scenarios‼️
‼️The table below shows how hemodynamics can alter management and assist with therapy in common clinical scenarios‼️

Current and more recent literature suggests that PA catheter utilization improves outcomes in advanced HF or cardiogenic shock. The figure below outlines the evolution of hemodynamics in HFrEF disease progression 

Lets delve deeper into the above figure:
As HF progresses, metabolic capacity gradually wanes and cardiac reserve is lost but rest perfusion remains normal
As HF progresses, metabolic capacity gradually wanes and cardiac reserve is lost but rest perfusion remains normal
As the SV wanes, compensatory mechanisms help maintain CO and BP. As the reserve fades, there is a supply-demand mismatch resulting in exacerbation and progression to low-output and shock states
In HFrEF, invasive hemodynamic assessments may be more helpful in advanced stages who present in a low-ouput, decompensated states where advanced therapies are being considered.
The low-output, decompensated state often discussed is Cardiogenic Shock (CS).
Defining CS: Primary myocardial insult decreasing CO to a level insufficient to maintain end-organ perfusion, resulting in pulmonary and/or venous congestion from increased cardiac filling pressures
Defining CS: Primary myocardial insult decreasing CO to a level insufficient to maintain end-organ perfusion, resulting in pulmonary and/or venous congestion from increased cardiac filling pressures
In CS there is a sudden impairment of CO this is usually poorly tolerated and results in pallor, cold extremities, and mental status changes; congestion is manifested by tachypnea and hypoxemia.
‼️Hemodynamic abnormalities may not be clinically apparent early on‼️
‼️Hemodynamic abnormalities may not be clinically apparent early on‼️
The predominant manifestation of CS is hypotension:
Key parameters:
-SBP <90 mmHg and MAP ≤60 mmHg for at least 30 min.
-Presence of hypotension demonstrated by physical exam or simple measures such as decreased urine output or increased lactate
Key parameters:
-SBP <90 mmHg and MAP ≤60 mmHg for at least 30 min.
-Presence of hypotension demonstrated by physical exam or simple measures such as decreased urine output or increased lactate
‼️Key point: Tissue hypoperfusion may be present w/o hypotension in the presence of endogenous vasoconstrictore reponses maintaining BP‼️
SCAI developed an integraded diagnostic approach to CS using both clinical and invasive hemodynamics to create a staging system for CS which recently has shown prognostic value:
Stage A: At Risk
Stage B: Beginning CS
Stage C: Classic CS
Stage D: Deteriorating
Stage E: Extremis
Stage A: At Risk
Stage B: Beginning CS
Stage C: Classic CS
Stage D: Deteriorating
Stage E: Extremis

Quick pause to link this Expert Consensus Update in SCAI SHOCK classification:
@SrihariNaiduMD @davebaran @SMHollenberg @seanvandiepen @shelleyhallmd @NavinKapur4 @SVRaoMD @thiele_holger @agtruesdell and the CSWG cc @ShashankSinhaMD @vbluml
jscai.org/article/S2772-…
@SrihariNaiduMD @davebaran @SMHollenberg @seanvandiepen @shelleyhallmd @NavinKapur4 @SVRaoMD @thiele_holger @agtruesdell and the CSWG cc @ShashankSinhaMD @vbluml
jscai.org/article/S2772-…

Many qualitative parameters are evaluated in CS patients
LV Cardiac Power Output (CPO) = CO x MAP/451
Cardiac Index
CVP
PCWP
RAP
‼️Neither CPO or CI are predictive of shock severity or mortality‼️
LV Cardiac Power Output (CPO) = CO x MAP/451
Cardiac Index
CVP
PCWP
RAP
‼️Neither CPO or CI are predictive of shock severity or mortality‼️
Hemodynamic phenotyping has been shown to have helpful in guiding therapeutic strategies until definitive therapy (transplant vs LVAD). Phenotyping algorithms relie on:
1. CPO
2. PA Pulsatility Index(assessement of RA and RV)
3. Filling Pressures
1. CPO
2. PA Pulsatility Index(assessement of RA and RV)
3. Filling Pressures

Mechanical circulatory support to pharmacologic support is utilized to prevent hemometabolic consequences of CS. So what is Hemometabolic shock?
–Congestion and malperfusion
–Decreased SVR due to vascular exhaustion
–Profound hypotension and hypoperfusion
–Congestion and malperfusion
–Decreased SVR due to vascular exhaustion
–Profound hypotension and hypoperfusion
Aquiring invasive hemodynamic data to confirm the diagnosis is critical in the team-based management of CS.
When considering destination therapy with transplant and LVAD for patients in endstage HF, invasive hemodynamics play an integral role in determining eligibility
A fixed precapillary PH is a relative contraindication to trasplantation––Pulmonary Vascular Resistence (PVR) >3.5-5.0 wood units (WU)
‼️High pulmonary pressures can precipitate acute right HF and death‼️
‼️High pulmonary pressures can precipitate acute right HF and death‼️
Patients with reversible percapillary PH can be amenable to transplant
-If pts with a baseline PVR >2.5 decreases to < 2.5 with nitroprusside therapy without systemic hypotension have decreased risk of acute RHF and death
-If pts with a baseline PVR >2.5 decreases to < 2.5 with nitroprusside therapy without systemic hypotension have decreased risk of acute RHF and death
Invasive exercise testing can directly measure supply (CO) and demand (VO2) assessing the ability of the heart ot match metabolic requirements during exercise.
–CO reserve limitation is defined by a supply-demand ratio <80% of expected
–CO reserve limitation is defined by a supply-demand ratio <80% of expected
However, noninvasive exercise testing is more often used to assess transplant candidacy and estimate CO reserve
⚠️50% of variability in peak VO2 is explained by AVO2-diff which cannot be measured noninvasively
⚠️50% of variability in peak VO2 is explained by AVO2-diff which cannot be measured noninvasively
Meaning, that patients with a low VO2 but normal CO reserve may respond better to exercise training than undergoing transplant or LVAD.
**Impairments in CO reserve more prognostic than peak VO2 in patients with advanced HFrEF undergiong transplant eval**
**Impairments in CO reserve more prognostic than peak VO2 in patients with advanced HFrEF undergiong transplant eval**
It is important to realize that some HF patients may have normal CO at rest, but virtually no CO reserve with exertion–indicating benefit from transplantation
Invasive diagnostics can play an integral role for patients being considered for transplant. Additionally, hemodynamics are equally as important when considering LVADs.
Early RHF after LVAD implantation remains a relatively common cause of post-LVAD morbidity and mortality, occurring in 9%–42% of cases.
@IRajapreyar @YevgeniyBr @AHajduczok
@IRajapreyar @YevgeniyBr @AHajduczok
Hemodynamic indices to help predict post-op early RHF:
Kussmaul's sign
Preop RAP (>15 mmHg)
Increased RAP/PCWP ratio (>0.54–0.63)
Decreased RV Stroke work index (<0.25 mmHg•L/m2)
Tachycardia
Low CI ( <2.2 L/min/m2)
Kussmaul's sign
Preop RAP (>15 mmHg)
Increased RAP/PCWP ratio (>0.54–0.63)
Decreased RV Stroke work index (<0.25 mmHg•L/m2)
Tachycardia
Low CI ( <2.2 L/min/m2)
An invasive hemodynamic parameter that has recently shown to predict early RHF in post-op LVAD patients is a decreased PA Pulsatility Index
PA pulsatility index = [PASP-PADP]/RAP
All parameters needed invasively!
PA pulsatility index = [PASP-PADP]/RAP
All parameters needed invasively!
Therefore, an integrated right heart assessment—incorporating clinical, imaging, and hemodynamic predictors of RHF—along with careful monitoring and early treatment of RHF, are well advised in the consideration and execution of LVAD surgery.
What about after LVAD implantation? Hemodynamics are equally as critical in management.
Hemodynamics enable precise assessments of CO, filling pressures, and afterload, which all significantly affect the very afterload-sensitive flow of blood through an LVAD
Hemodynamics enable precise assessments of CO, filling pressures, and afterload, which all significantly affect the very afterload-sensitive flow of blood through an LVAD
Hemodynamics, when added to echocardiography, also improve diagnostic accuracy when considering LVAD complications such as pump thrombosis, because pump compromise may manifest in LV pressure and CO changes not apparent on imaging.
40% of patients with an LVAD have suboptimal RAP and PCWP, even after clinical/ echocardiographic-based optimization.
But, PA catheterization can help make optimization more accurate
But, PA catheterization can help make optimization more accurate
Hemodynamic-guided ramp studies, where both RHC and echo measurements are repeated after successive LVAD speed increases, can improve filling pressure optimization, direct subsequent medical interventions, and potentially decrease HF hospitalizations
ahajournals.org/doi/10.1161/CI…
ahajournals.org/doi/10.1161/CI…

Finally, current generation LVADs maintain a static speed, which limits CO reserve and peak VO2, and invasive exercise testing may allow for more refined estimates of the ideal LVAD speed that optimizes both resting and exercise flow.
>50% of patients with HF suffer from HFpEF, but diagnosis of HFpEF can be difficult unless it is overt with the clinical manifestations, and noninvasive evidence of congestion and diastolic dysfunction at rest.
Thus, noninvasive diagnostic criteria applied at rest miss a large proportion of HFpEF patients. Why?
Before abnormalities manifest at rest, the left heart in HFpEF loses its ability to maintain low filling pressures while trying to meet the increased demands of exercise
Before abnormalities manifest at rest, the left heart in HFpEF loses its ability to maintain low filling pressures while trying to meet the increased demands of exercise
Owing to the poor sensitivity of clinical and noninvasive diagnostics, invasive hemodynamic testing plays a major role in the optimal evaluation of HFpEF.
–RHC can diagnose increased left-sided filling pressures at rest while concurrently ruling out group I PH
–If indicated, endomyocardial biopsy performed at time of RHC can rule out cardiac amyloidosis.
–If indicated, endomyocardial biopsy performed at time of RHC can rule out cardiac amyloidosis.
Hemodynamics during exercise testing in HFpEF is even more useful as the variable defining HF are directly measured!
HFpEF is defined invasively during supine exercise as a peak exercise PCWP of 25 mmHg or greater––current diagnostic guideline definition
HFpEF is defined invasively during supine exercise as a peak exercise PCWP of 25 mmHg or greater––current diagnostic guideline definition
Complementary hemodynamic pattern indicative of HFpEF:
Measuring PCWP as a function of CO at regular intervals during exercise
––A PCWP/CO slope of >2 mmHg•min/L has been predictive of outcomes
Measuring PCWP as a function of CO at regular intervals during exercise
––A PCWP/CO slope of >2 mmHg•min/L has been predictive of outcomes
Alternatives to invasive exercise testing:
1. Saline loading
2. Leg raise hemodynamics
3. Exercise Echocardiography
1. Saline loading
2. Leg raise hemodynamics
3. Exercise Echocardiography
1. Saline Loading; Simple, but requires catheterization
–Sensitivity is inferior to exercise testing
–Sensitivity is inferior to exercise testing
2. Passive Leg Raise Hemodynamics
–Increase in the PCWP with a passive leg raise has been shown to provide good discrimi- nation of HFpEF from noncardiac dyspnea, but pro- vides less physiologic information.
–Increase in the PCWP with a passive leg raise has been shown to provide good discrimi- nation of HFpEF from noncardiac dyspnea, but pro- vides less physiologic information.
3. Exercise Echocardiography
Exercise echocardiography shows promise as a noninvasive method, but diagnostic accuracy is still unclear
Exercise echocardiography shows promise as a noninvasive method, but diagnostic accuracy is still unclear
Assessing pre-test probability of HFpEF using the H2FPEF Score vs HFA-PEFF Score thanks to @yreddyhf and colleagues helped develop a step-wise approach in HFpEF diagnostics to help determine the need for invasive hemodynamic testing in patients with unexplained dyspnea 

The H2FPEF and HFA-PEFF Scoring systems show that those with low and high probability can be ruled out and in, respectively, while those with intermediate probability (score 2-5) can go on to invasive hemodynamic exercise testing
What about hemodynamics in pulmonary hypertension? PH has been defined as a mPAP of 25 mmHg or higher, but recent studies have revealed that a mPAP between 21-24 mmHg is associated with increased mortality
@RyanTedfordMD @jeanlucvachiery
@RyanTedfordMD @jeanlucvachiery
However, to diagnose group 1 PH (precapillary or pulmonary arterial hypertension,PAH), a PVR of 3 or more WU and a PCWP of less than 15 mm Hg must also be present. Once PAH is diagnosed, vasoreactivity testing should be done because it influences prognosis and treatment
Vasoreactivity via inhaled nitric oxide or IV epoprostenol is present if mPAP decreases by >10 mmHg to <40 mmHg without a decrease in CO
Group 2 PH is diagnosed when the mPAP is greater than 20 mm Hg and the PCWP is 15 mm Hg or higher and is seen in at least 50%-80% of patients. Subcategories of gorup 2 PH include
1. Isolated post-capillary PH
2. Combined precapillary and post-capillary PH
1. Isolated post-capillary PH
2. Combined precapillary and post-capillary PH
1. Isolated post-capillary PH when the PVR is less than 3 WU
2. Combined precapillary and post-capillary PH when the PVR is 3.0 or more WU, where the mPAP is increased out of proportion to left heart disease
2. Combined precapillary and post-capillary PH when the PVR is 3.0 or more WU, where the mPAP is increased out of proportion to left heart disease
Combined precapillary and post-capillary PH have distinct hemodynamic profiles
–Greater RV Dysfunction
–Ventricular interdependence
–More severe exercise limitaiton
–Greater risk of death
–Greater RV Dysfunction
–Ventricular interdependence
–More severe exercise limitaiton
–Greater risk of death
In PH, hemodynamic indices reflecting RHF below predict poor functional capacity and outcomes in group 1 and 2 PH
-Increased RAP
-Decreased SV
-Kussmaul's sign
-Increased RAP
-Decreased SV
-Kussmaul's sign
PVR is related to PA compliance in a hyperbolic fashion
⚠️PCWP Increases results in lower PA compliance at any given PVR
⚠️PA compliance decrease is highly prognostic in group 2 PH
⚠️PCWP Increases results in lower PA compliance at any given PVR
⚠️PA compliance decrease is highly prognostic in group 2 PH
Distinguishing group 1 from group 2 PH is critical
-Group 1 has multiple effective therapies
-Group 2 with no proven direct therapies
The lynchpin for this distinction relies on accurate assessment of PCWP.
-Group 1 has multiple effective therapies
-Group 2 with no proven direct therapies
The lynchpin for this distinction relies on accurate assessment of PCWP.
The wedge saturation may be particularly useful; wedge saturation measurements reclassified PH grouping in 11.8% of patients when compared with diagnoses based on PCWP measured without saturation assessment.
Thus, identifying PH group is critical in management and invasive hemodynamic testing to identify PCWP and obtaining wedge saturations can be useful in distinguishing group 1 and group 2 PH
In addition to group 1 and 2 PH, there lies another category–Exercise-induced PH (EiPH)
Defined initially by an exercise mPAP of >30 mmHg
Unfortunatley this has been removed from guidelines, so what now?
Defined initially by an exercise mPAP of >30 mmHg
Unfortunatley this has been removed from guidelines, so what now?
Two criteria have been proposed:
1. EiPH as an exercise mPAP/CO slope of greater than 3 (mmHg•min)/L
2. EiPH when both the mPAP is greater tan 30 mm Hg and the total pulmonary resistance (mPAP/CO) is more than 3 WU at exercise peak
1. EiPH as an exercise mPAP/CO slope of greater than 3 (mmHg•min)/L
2. EiPH when both the mPAP is greater tan 30 mm Hg and the total pulmonary resistance (mPAP/CO) is more than 3 WU at exercise peak
To distinguish EiPH from HFpEF, invasive hemodynamic measurements of PCWP during exercise is necessary
Recent literature suggests that patients with EiPH have poorer clinical outcomes, thus early diagnosis is critical to management
Recent literature suggests that patients with EiPH have poorer clinical outcomes, thus early diagnosis is critical to management
Finally, invasive hemodynamics play a crucial role in distinguishing constrictive pericarditis from primary myocardial disease
Both characterized by increased right and left sided filling pressures with steep Y-descents, frequently positive Kussmaul signs and dip-and-plateau sign in the ventricles
But simultaneous comparison of RV–LV and LV–PCWP pressure tracings distinguish myocardial from pericardial
But simultaneous comparison of RV–LV and LV–PCWP pressure tracings distinguish myocardial from pericardial
In constriction, LV and RV systolic pressure changes during respiration are discordant, or 180° out of phase with one another, due to enhanced ventricular interdependence.
Additionally, intrathoracic–intracardic dissociation results in the mean pressure gradient between PCWP and LV during diastole to decreases by ≥5 mmHg
In restrictive myocardial disease, LV and RV pressures change in phase and there is no dissociation or enhanced ventricular interdependence.
The value of invasive hemodynamics has re-emerged front and center, playing a crucial role in the scope of the HF practice. Understanding
If you made it this far - consider yourself a hemodynamics purist (and maybe even an expert now!) Tag your friends who would love this!!
@YevgeniyBr @AndrewJSauer @RyanTedfordMD @DrMarthaGulati @noshreza @EddyGumbs @CheryGodefroy @JJheart_doc @PPirlamarla_MD @KashviGupta
@YevgeniyBr @AndrewJSauer @RyanTedfordMD @DrMarthaGulati @noshreza @EddyGumbs @CheryGodefroy @JJheart_doc @PPirlamarla_MD @KashviGupta
@vbluml @QuentinYoumans @AditiNayakMD @ManingJennifer @robmentz @dranulala @ShelleyZieroth @DBelardoMD @JasonKatzMD @JonathanDavisHF @rachkataria @SantosGallegoMD @JumeanMarwan @Nikhil15 @MarkBelkinMD @FudimMarat @DrRossMasood @DRJoycewald @SarasVallabhMD @Jcontreras75
@Abraham_Jacob @ajaysmd @fsheikh22 @ranga1949 @baileyannRN @DLHollandMD @HeartOTXHeartMD @docbhardwaj @DukeHFDoc @hfdocbhimaraj @LAuroraMD @MollySilkowski @CardioNerds @amitalamMD @AmitGoyalMD @ThomasMDas @KatieV_MD @AndrewHigginsMD @RanLeeMD @av1hs @HanCardiomd @DrNasrien
@EiranGorodeski @preventfailure @manreetkanwar @amorrismd @crfheart @ISHLT @AminYehyaMD @HFSA @ACCinTouch @American_Heart @JavedButler1 @MarkDrazner @jteerlinkmd @NirUrielMD @JonGrinsteinMD @rcogswell_umn @DrJennHaythe @MaryjaneFarrMD @quin_denfeld @colleenkmac @ankeetbhatt
@mvaduganathan @rcstarling @DrQuinnCapers4 @ersied727 @ParagGoyalMD @heartofthemater @_adevore @JeffTeuteberg @TomCascinoMD @JHMontfort10 @BiykemB @karanpdesai @a_h_ghoneem @mpsotka @MKIttlesonMD @gmac78 @AliNsairMD @yaleHFdoc @gregorytgibson @EugeneStorozyn6 @BrenerMickey
@DavidWienerMD @TJHeartFellows @JeffHsuMD @mmamas1973 @drjohnm @drkaushikjain @JanMGriffin @BrettSperryMD @MichaelNassifMD @KevinShahMD @WilcoxHeart @VorovichHeartMD @YasMoayedi @StavrosDrakos @SusanJosephMD @AlbertHicksMD
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter



