Excited to share my first scientific manuscript out now on @biorxivpreprint, where we present the first ever #cryoEM structure of the iron #nitrogenase complex. Let me tell you about our newest insights into the mystery of biological nitrogen fixation: doi.org/10.1101/2023.0… 

Nitrogen is an essential building block for all life on Earth. However, activating molecular nitrogen (N2) and utilising it for biological means is a tremendous challenge due to the inert chemical properties of N2. How does nature deal with this?
Evolution has created a sophisticated class of metalloenzymes referred to as nitrogenases. Utilising some of the most complex metallocofactors known to nature, nitrogenases can reduce atmospheric N2 to bioavailable ammonia under ambient conditions.
Three nitrogenase isoforms are known to date: The molybdenum (Mo), vanadium (V) and iron (Fe) nitrogenase. From these, the Fe nitrogenase was the latest isoform to be discovered and is consequently the least understood, an issue that we sought to address. 
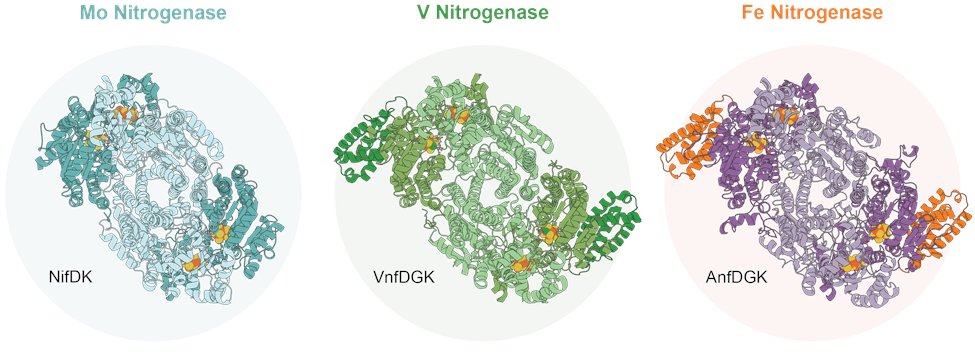
Using #Rhodobacter capsulatus, we recombinantly expressed and purified the Fe nitrogenase to solve a 2.35 Å #cryoEM structure of the enzyme complex. Thereby, we present a snapshot of the active Fe nitrogenase, setting the basis for a detailed analysis of its molecular mechanism. 

In contrast to the Mo isoform, the V and Fe nitrogenases contain an extra subunit, the G-subunit, whose function remains elusive up to date. Based on our structure we propose three potential functions of the Fe nitrogenase G-subunit (AnfG):
First, we identified several amino acid residues of the Fe nitrogenase G-subunit (AnfG) that are in direct contact with the bound reductase component. Thus, we speculate that AnfG contributes to the specificity of the reductase component binding process. 

Second, we identified the two likeliest N2 substrate channels leading to the nitrogenase active site cofactor (FeFeco), which both initialise within the G-subunit. Hence, we suggest a regulatory function of AnfG in modulating substrate accessibility to the nitrogenase. 

Third, our data suggest an involvement of AnfG in stabilising the active site cofactor (FeFeco). This conclusion is based on the fact that AnfG is positioned right on top of the alphaIII domain, which is involved in the insertion and stabilisation of the active site cofactor. 

We further analysed the Fe nitrogenase by comparing it to the other nitrogenase isoforms, whose structures have been solved previously. Overlaying the structures, we noticed that one half of the Fe and V nitrogenase appeared to be kinked relative to the Mo nitrogenase.
Comparing the symmetric interfaces of the nitrogenases, we identify two structural features that might be responsible for this observation: An elongated C-terminus of the Fe nitrogenase D-subunit and a short insertion in the Mo nitrogenase K-subunit. 

Recently, the two halves of the nitrogenase enzyme were proposed to function in a cooperativity like mechanism. Given the divergent interactions of the two halves described here, we expect a distinct mechanism for the Fe nitrogenase that could explain its unique reactivities.
In summary, our work provides the foundation to rationally modify and test the differences among the nitrogenase isoenzymes to provide new insights on the catalytic profiles of the three nitrogenase isoforms.
Lastly, I´d like to thank my closest collaborator and friend @schulluc who shares the first authorship with me on this paper as well as my mentor @RebeleinLab. I am equally grateful to all authors and collaborators @Trematode12, Niels, Simone and @erblabs. Thank you for reading.
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter



