$TTOO A thread for current/future investor:
1.) $TTOO Investor presentation on 08/15/23 looked very promising, take a look at all the subsections covered by them in the presentation.
1.) $TTOO Investor presentation on 08/15/23 looked very promising, take a look at all the subsections covered by them in the presentation.
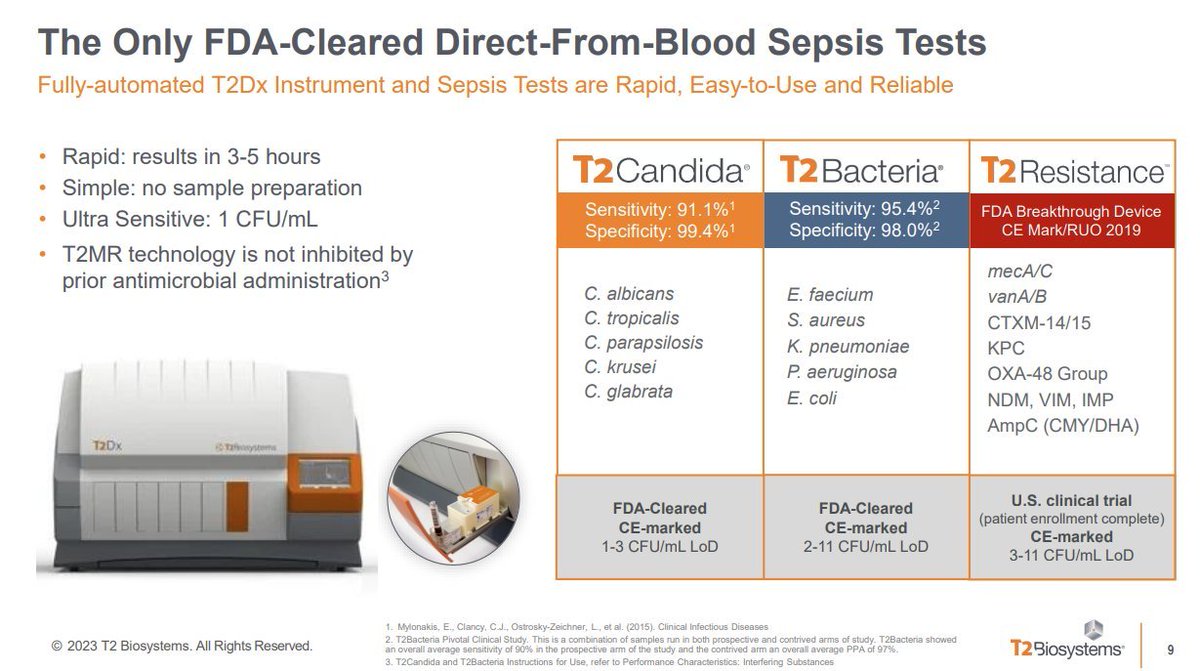
2.) $TTOO's T2Dx Instrument & Sepsis Tests are rapid (3-5hr results), simple (no prep), & ultra-sensitive (1 CFU/mL). T2MR tech isn't inhibited by prior antimicrobials. A reliable & easy-to-use advancement in diagnostics. 
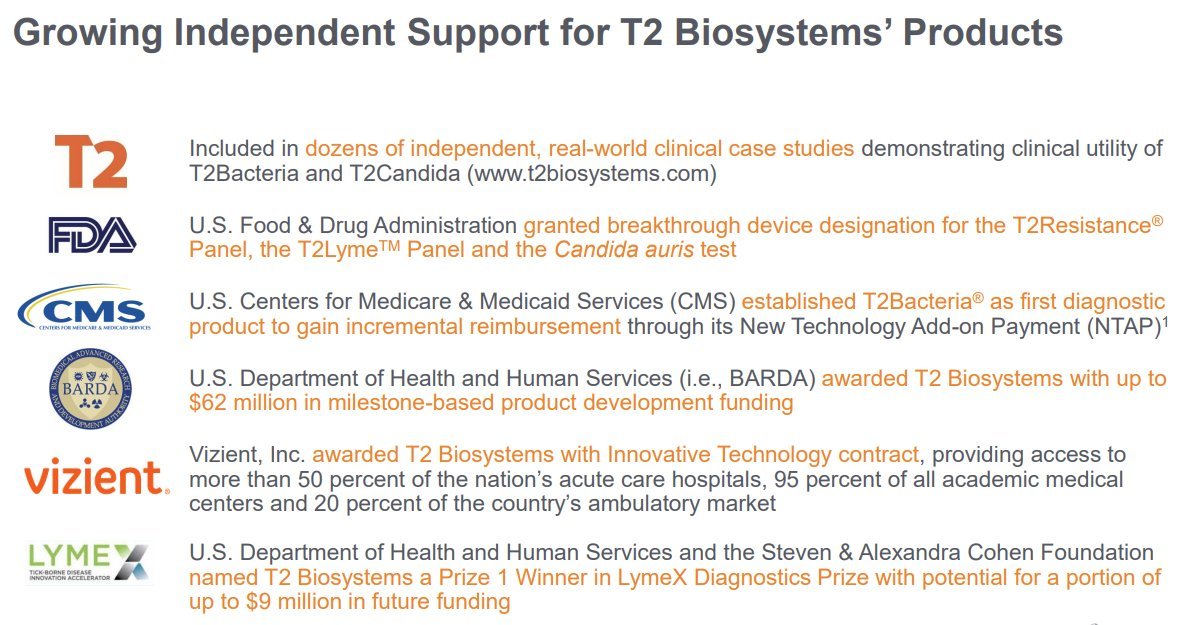
3.) Growing Independent Support for T2 Biosystems’ Products: Included in dozens of independent, real-world clinical case studies demonstrating clinical utility of T2Bacteria and T2Candida (). FDA granted breakthrough device designation for T2Resistance® Panel, T2Lyme™ Panel, and Candida auris test. CMS established T2Bacteria® as first diagnostic product to gain incremental reimbursement through NTAP. HHS (i.e., BARDA) awarded T2 Biosystems with up to $62 million in milestone-based product development funding. Vizient, Inc. awarded T2 Biosystems with Innovative Technology contract, providing access to more than 50% of the nation’s acute care hospitals, 95% of all academic medical centers, and 20% of the country’s ambulatory market. HHS and the Steven & Alexandra Cohen Foundation named T2 Biosystems a Prize 1 Winner in LymeX Diagnostics Prize with potential for a portion of up to $9 million in future fundingt2biosystems.com
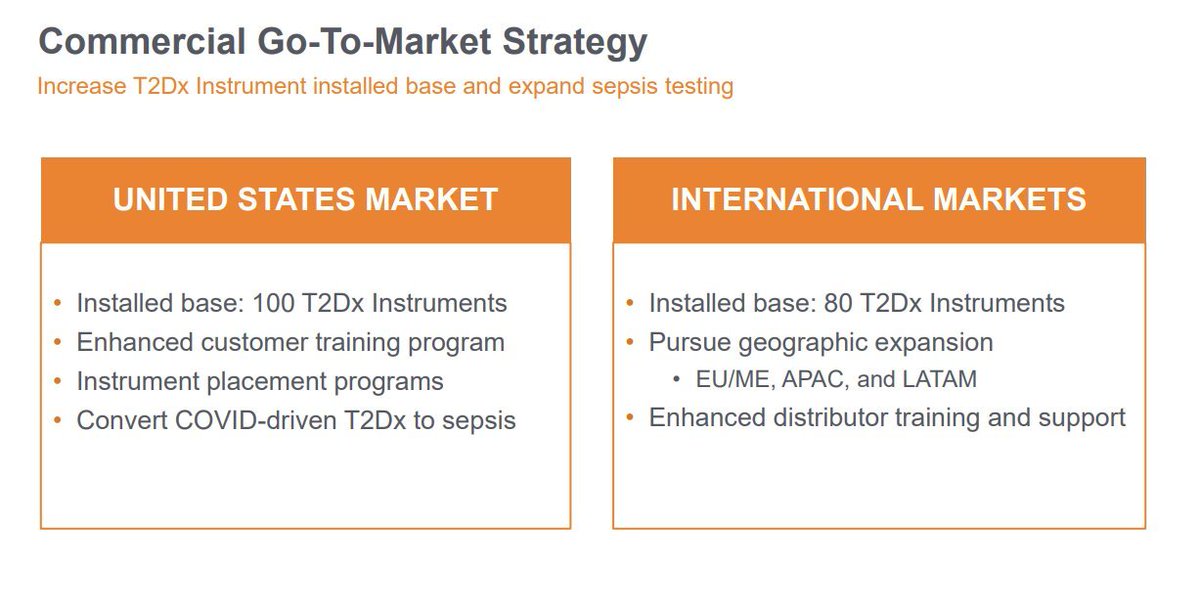
4.) Commercial Go-To-Market Strategy for $TTOO: In the U.S., there's an installed base of 100 T2Dx Instruments, enhanced customer training, instrument placement programs, and a focus on converting COVID-driven T2Dx to sepsis. Internationally, with 80 T2Dx Instruments installed, the strategy includes pursuing geographic expansion in EU/ME, APAC, and LATAM, along with enhanced distributor training and support.
5.) Menu Expansion Initiatives for $TTOO's FDA Cleared T2Dx Instrument include the T2Biothreat Panel, designed to detect 6 biothreat pathogens in 3-5 hours, with FDA submission filed in May 2023. The T2Resistance Panel detects 13 antibiotic resistance genes and has completed U.S. clinical trials. The Acinetobacter baumannii Test aims to detect a common bacterial pathogen, with FDA submission planned for 2H 2023. The Candida auris Test, developed in collaboration with CDC, detects a global health threat in 3-5 hours. The T2Lyme Panel, designed to detect the bacteria causing Lyme disease, has received FDA Breakthrough Device Designation and LymeX prize. All tests emphasize rapid results without waiting days for blood culture

6.) Operational Objectives for $TTOO: The focus is on achieving on-time delivery targets, improving product gross margins, and achieving ISO recertification. Efforts are also being made to reduce operating costs, transfer new products from R&D, scale manufacturing processes, and implement the Oracle ERP system. A comprehensive approach to enhancing efficiency and innovation

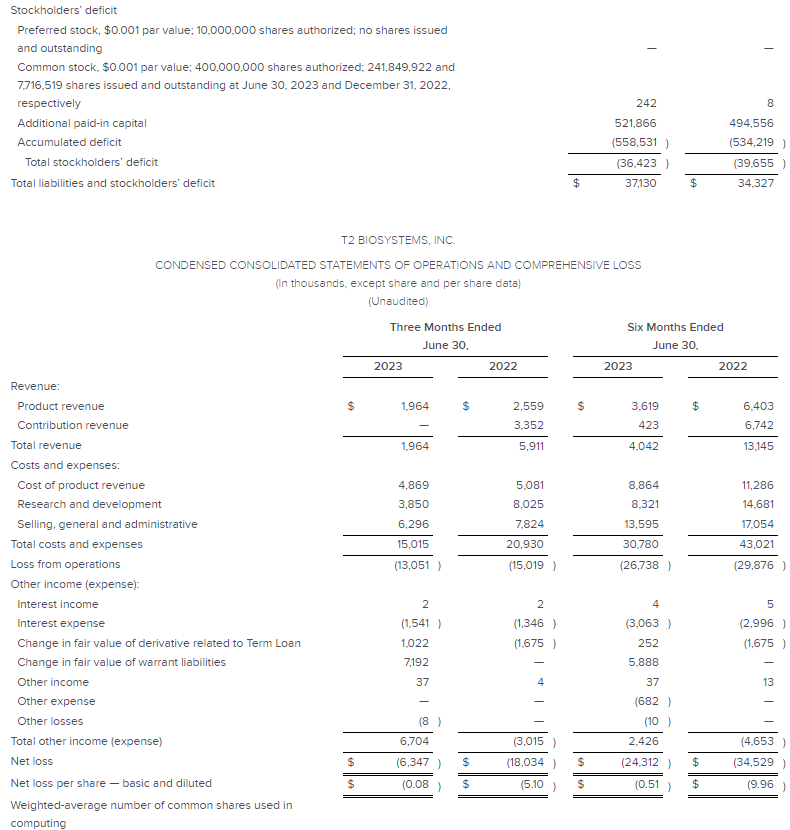
7.) Concluding this thread with a financial summary for $TTOO: Total Revenue of $2.0 million, including a record quarterly sepsis test panel revenue in Q2 with a 7% Y-o-Y increase. Product Revenue also at $2.0 million, with the 2nd largest sepsis-driven T2Dx Instrument order in Poland. R&D Revenue at $0.0 million, with key achievements like FDA Breakthrough Device designation for Candida auris test. Cash Balance stands at $16.1 million, strengthened by CRG debt-to-equity conversion and capital raise. Make your decision based on this thread at your own discretion. This isn't financial advice, but an overlook of what's going on at T2 :

8.) Additionally, $TTOO's Next Generation Platform, funded under a milestone-based BARDA contract valued up to $62 million, is paving the way for advanced diagnostics. The Comprehensive Sepsis Panel is designed to detect over 95% of all bloodstream infections caused by bacterial and Candida species, along with antibiotic-resistant markers, in just ~3 hours. The Next Generation Instrument, designed to be fully automated and random access like the FDA-cleared T2Dx Instrument, will work in tandem with the sepsis panel to detect an increased number of pathogens and antibiotic resistance genes from a single whole blood sample.

• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter