Why is it so hard to investigate viral infection in the human brain?
At the beginning of the #COVID19 pandemic, many studies were categorical, affirming that the virus had no neurotropism and could not infect brain cells. Many people must realize that working with postmortem human brain tissue offers many challenges for viral studies.
Each human dies in different circumstances, and having the same time interval to start preparing the brain for analysis is impossible. Not only is the time variable, but it is usually very long compared to the controlled environment when working with animal models in a laboratory.
In addition, several experimental models of neurotropic viral infections have shown that viruses are usually quickly cleared from the brain despite the initial fast spreading. Given that most autopsy studies inevitably have long, variable postmortem intervals before tissue processing, especially during the pandemic, infected neurons from highly connected olfactory regions may be dying early and thus evading detection.
Detecting active viruses in the brain, plasma, and imaging biomarkers can help us shed light on early neurological events. Many biomarker studies have reported that cognitive decline is a common finding, and even non-severe COVID-19 is associated with early-onset cognitive decline and fluid biomarkers alteration, such as higher plasma Neurofilament concentration.
There are other challenges to face regarding having good animal models for studying #SARSCoV2. The mouse ACE2 receptor does not effectively bind the viral spike protein, and Labs had to create transgenic mice models expressing human Ace2 to visualize the viral damage in the brain and other regions. I work with monkeys, which offers a substantial advantage since they can be naturally infected with SARSCoV2 and experience disease progression similar to humans. However, I am aware that most Labs don't have access to newly developed transgenic mice models or primates, and these factors contribute to slowing down and misguiding research progression in this field.
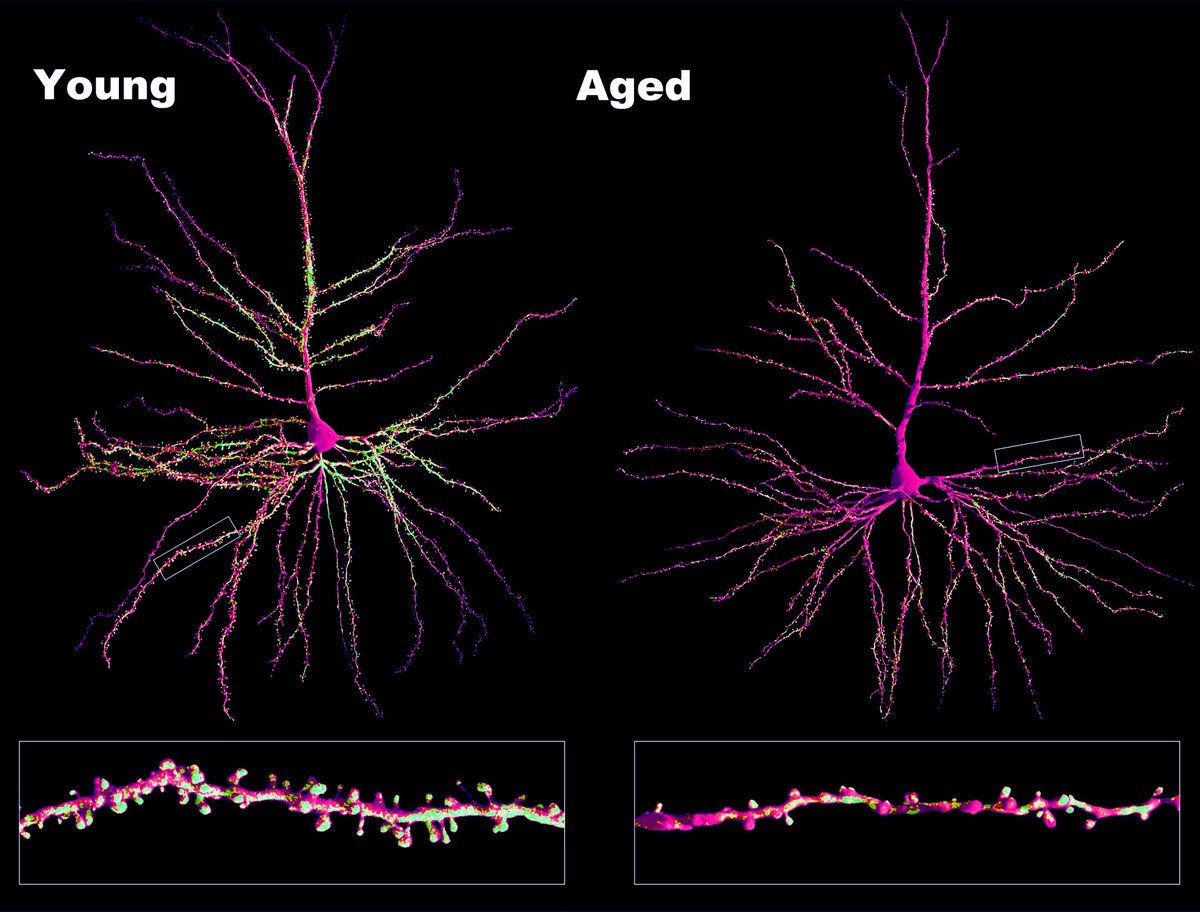
For me, the damage and infection in the brain is directly visible in the microscope. We see that SARSCoV2 infects almost all cell types in the brain. We detect neurons and glia cells containing abundant Ace2 receptors and observe Spike protein (🟣) binding to them. We also observe viral double-stranded RNA (dsRNA🔴) concentrate exclusively in the cell body of neurons (neuronal protein 🟢). That means the virus can infect many different brain cell types but prefers using neurons to replicate and propagate in the brain. This affinity is likely because replicating inside the neuron and using axonal transport for propagation is the fastest mechanism for a virus to spread in the brain. Beaded, fragmented, and degenerating axons are common findings in COVID-19-infected brains; consequently, substantial degradation of myelin and neuroinflammation is also visible.
Neurovirology is a recent field and is full of many challenges. Detection of single viral particles in axons or viral reproduction apparatus within neurons in brain regions remains a tremendous challenge. However, not acknowledging that SARSCoV2 infects the brain and causes substantial behavioral and cognitive alterations is denialism and irrational action to avoid the uncomfortable truth that the scientific method suggests.
History has shown that this contributes to nothing other than slowing progress and discoveries, and this concept is termed the Semmelweis reflex. We need to keep fighting against it.
At the beginning of the #COVID19 pandemic, many studies were categorical, affirming that the virus had no neurotropism and could not infect brain cells. Many people must realize that working with postmortem human brain tissue offers many challenges for viral studies.
Each human dies in different circumstances, and having the same time interval to start preparing the brain for analysis is impossible. Not only is the time variable, but it is usually very long compared to the controlled environment when working with animal models in a laboratory.
In addition, several experimental models of neurotropic viral infections have shown that viruses are usually quickly cleared from the brain despite the initial fast spreading. Given that most autopsy studies inevitably have long, variable postmortem intervals before tissue processing, especially during the pandemic, infected neurons from highly connected olfactory regions may be dying early and thus evading detection.
Detecting active viruses in the brain, plasma, and imaging biomarkers can help us shed light on early neurological events. Many biomarker studies have reported that cognitive decline is a common finding, and even non-severe COVID-19 is associated with early-onset cognitive decline and fluid biomarkers alteration, such as higher plasma Neurofilament concentration.
There are other challenges to face regarding having good animal models for studying #SARSCoV2. The mouse ACE2 receptor does not effectively bind the viral spike protein, and Labs had to create transgenic mice models expressing human Ace2 to visualize the viral damage in the brain and other regions. I work with monkeys, which offers a substantial advantage since they can be naturally infected with SARSCoV2 and experience disease progression similar to humans. However, I am aware that most Labs don't have access to newly developed transgenic mice models or primates, and these factors contribute to slowing down and misguiding research progression in this field.
For me, the damage and infection in the brain is directly visible in the microscope. We see that SARSCoV2 infects almost all cell types in the brain. We detect neurons and glia cells containing abundant Ace2 receptors and observe Spike protein (🟣) binding to them. We also observe viral double-stranded RNA (dsRNA🔴) concentrate exclusively in the cell body of neurons (neuronal protein 🟢). That means the virus can infect many different brain cell types but prefers using neurons to replicate and propagate in the brain. This affinity is likely because replicating inside the neuron and using axonal transport for propagation is the fastest mechanism for a virus to spread in the brain. Beaded, fragmented, and degenerating axons are common findings in COVID-19-infected brains; consequently, substantial degradation of myelin and neuroinflammation is also visible.
Neurovirology is a recent field and is full of many challenges. Detection of single viral particles in axons or viral reproduction apparatus within neurons in brain regions remains a tremendous challenge. However, not acknowledging that SARSCoV2 infects the brain and causes substantial behavioral and cognitive alterations is denialism and irrational action to avoid the uncomfortable truth that the scientific method suggests.
History has shown that this contributes to nothing other than slowing progress and discoveries, and this concept is termed the Semmelweis reflex. We need to keep fighting against it.

• • •
Missing some Tweet in this thread? You can try to
force a refresh