Into the weeds:
One reason it is particularly frustrating that full metadata has not been shared for genome sequences my colleagues and I have assembled from raw sequence read data released by @USDA / @USDA_APHIS, is that without those dates...
One reason it is particularly frustrating that full metadata has not been shared for genome sequences my colleagues and I have assembled from raw sequence read data released by @USDA / @USDA_APHIS, is that without those dates...
it is not possible to test some really important hypotheses.
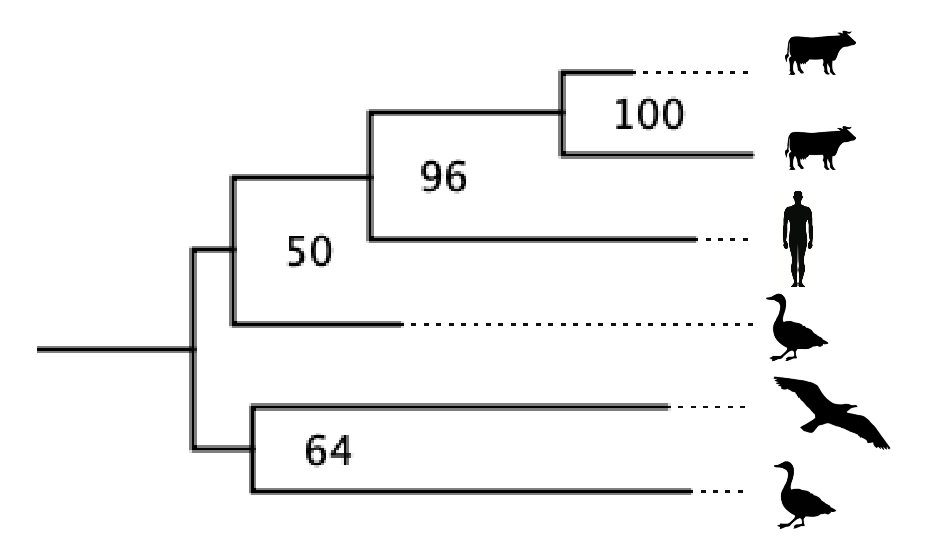
Years ago, staring long enough (weeks) at evolutionary trees of all 8 flu A genomes segments that stored on my kitchen table, it finally occurred to my brain that you can't just assume that these viruses evolve...
Years ago, staring long enough (weeks) at evolutionary trees of all 8 flu A genomes segments that stored on my kitchen table, it finally occurred to my brain that you can't just assume that these viruses evolve...
at the same rate in each host species. Andrew Rambaut and I devised a "local molecular clock" to allow the virus molecular clock to tick at a different rates in each host species.
Andrew, my student Guan-Zhu Han, and I published a paper showing that when this empirical reality was included in our models, the picture of the deep history of these viruses, which had been hopelessly confusing, snapped into focus:
nature.com/articles/natur…
nature.com/articles/natur…
My concern at the moment is that H5N1 might evolve at a different rate in cattle than in birds. And until we have the sampling dates for the cattle viruses, we won't know if it is faster or slower (or the same) in cattle and birds.
And that means the outbreak could be somewhat older or younger than our current estimates.
We want to be able to understand, ASAP, how this cattle outbreak originated and what is full consequences might be.
And we have at least one hand tied behind our back at the moment.
We want to be able to understand, ASAP, how this cattle outbreak originated and what is full consequences might be.
And we have at least one hand tied behind our back at the moment.
• • •
Missing some Tweet in this thread? You can try to
force a refresh