Just out: mRNA COVID-19 vax do not establish in long-lived plasma cells; could account for faster waning vs. infection. Other mechanisms still protect durably vs. severe illness, but improving durability is important. Adjuvants (like in Novavax) may help.
nature.com/articles/s4159…
nature.com/articles/s4159…
My previous thoughts on the preprint here.
h/t @atranscendedman
h/t @atranscendedman
https://x.com/Daniel_E_Park/status/1766079718854455681
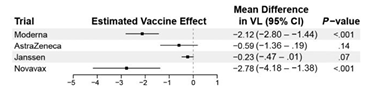
The paper shows that compared with influenza and tetanus vaccination, the mRNA COVID-19 vaccines were unable to imprint LLPCs. Also, serum IgG levels correlated well with bone marrow LLPC levels for flu and tetatnus, but not for SARS-CoV-2. 
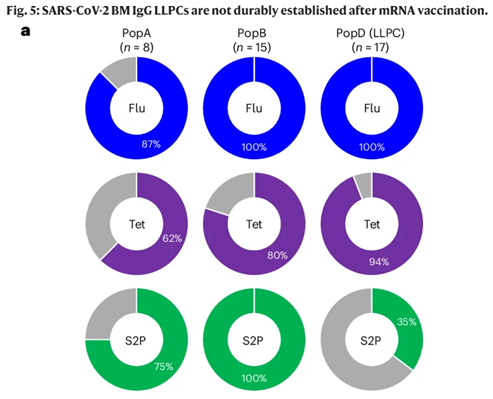
The discussion section is well-written, and contemplates whether this is unique to the mRNA platform or to the spike protein, since durable protection to other coronaviruses is also relative short-lived. I think it’s both.
The authors conclude with a call to explore different vaccination schedules, vaccine adjuvants, or delivery systems. 

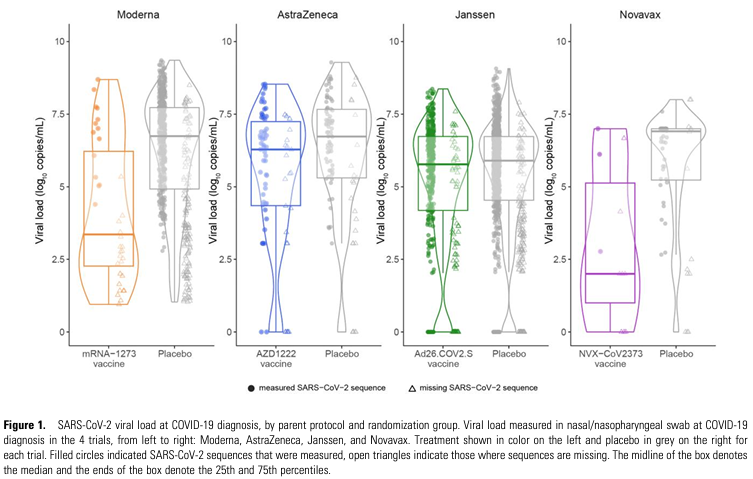
Naturally, those with experience working with vaccines know a primary purpose of adjuvants is to enhance durability. The existing Novavax COVID-19 vaccine uses a saponin adjuvant and I have hypothesized that it could be a key factor in why it may provide more durable protection.
Enhanced durability via adjuvants has been shown, among others, for Shingles (Shingrix vs. Zostavax). The adjuvanted platform lasts 7-10 years vs. non-adjuvanted Zostavax which dropped to 50% efficacy by year 2.
bmj.com/content/383/bm…
academic.oup.com/ofid/article/9…
bmj.com/content/383/bm…
academic.oup.com/ofid/article/9…
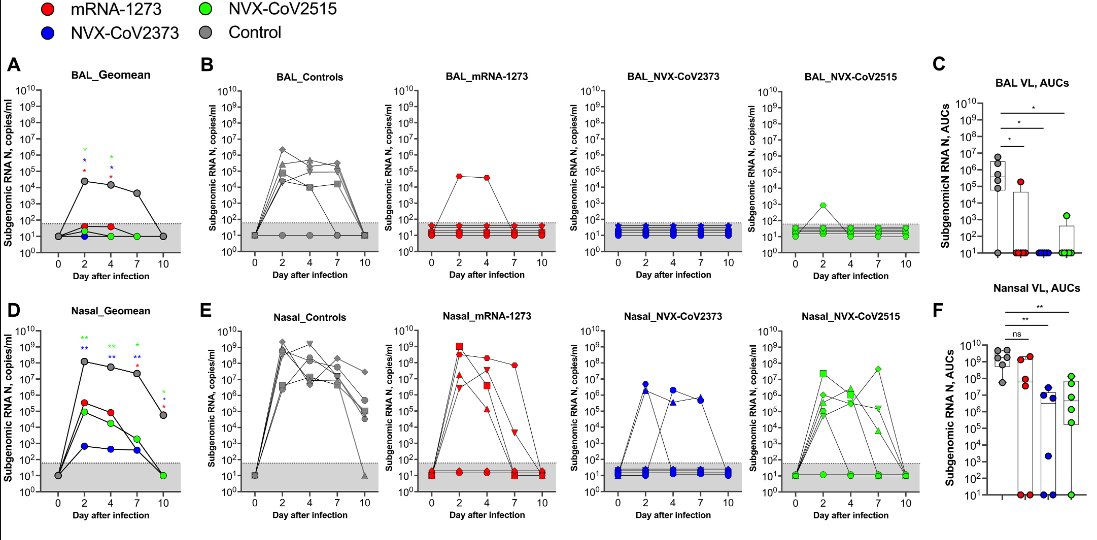
And multiple animal models with the Novavax platform have demonstrated LLPCs in addition to blunting of upper respiratory tract viral load with the Novavax vaccine.
nature.com/articles/s4154…
science.org/doi/10.1126/sc…
nature.com/articles/s4154…
science.org/doi/10.1126/sc…
Other COVID-19 vaccines in development using similar saponin-based adjuvants have also demonstrated induction of LLPCs in bone marrow. This suggests the spike protein may present challenges to B cell activation, but can be partially overcome via adjuvants.
science.org/doi/10.1126/sc…
science.org/doi/10.1126/sc…
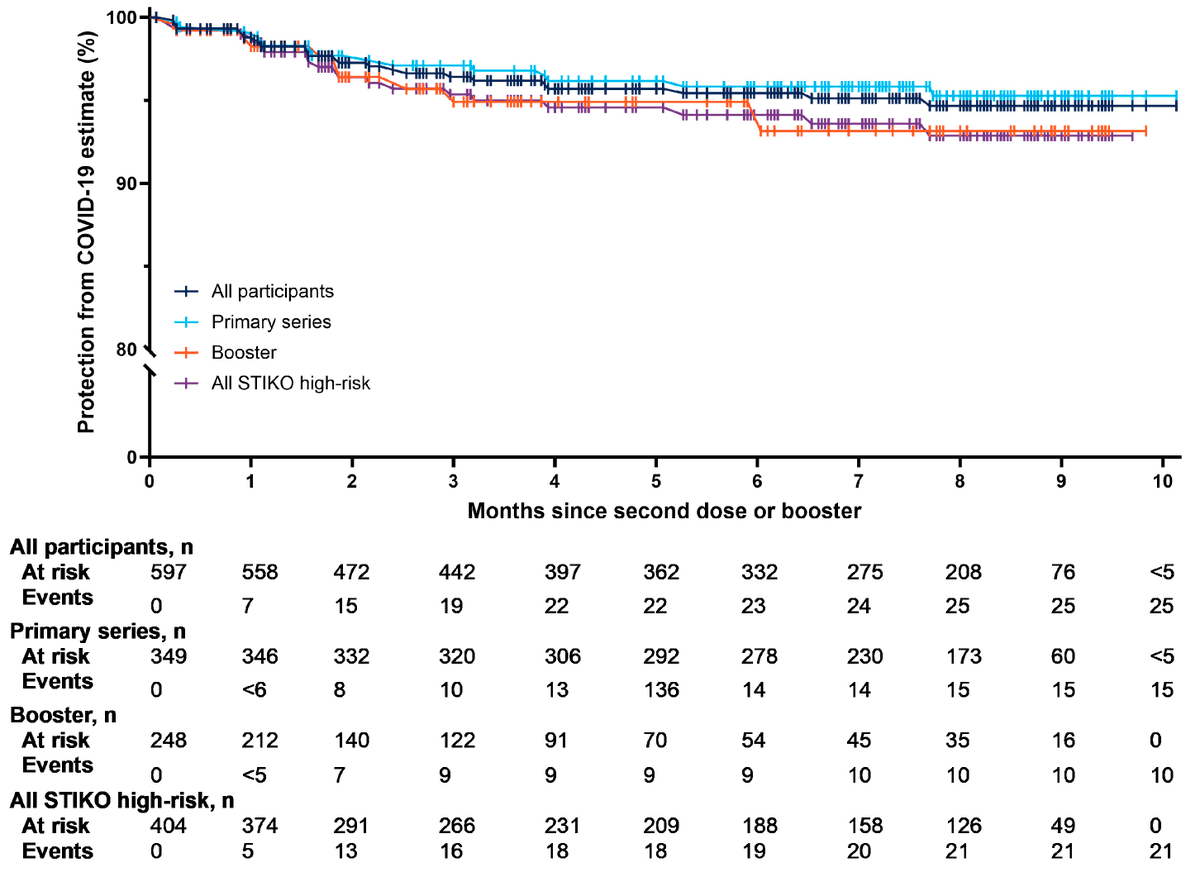
Finally, the vaccine trials showed good durability (>75% at 6-months from Delta period in vaccine trial using original strain vaccine). And population based studies suggest good long-term durability with NVX
academic.oup.com/cid/article/79…
jamanetwork.com/journals/jaman…
mdpi.com/2076-393X/12/4…

academic.oup.com/cid/article/79…
jamanetwork.com/journals/jaman…
mdpi.com/2076-393X/12/4…
mRNA COVID-19 vaccines have played a critical role in blunting the impact of SARS-CoV-2 throughout the last 5 years. But since the virus circulates throughout the year, it is critical to 1) improve air quality and/or mask in high-exposure areas, 2) improve durability of vaccines.
On #2, human studies of NVX LLPCs & durability are needed to see if a solution already exists. Often animal models don't translate to humans, & durability studies are challenging due to pre-existing immunity, variants, etc. But early suggestions certainly warrant further studies
• • •
Missing some Tweet in this thread? You can try to
force a refresh