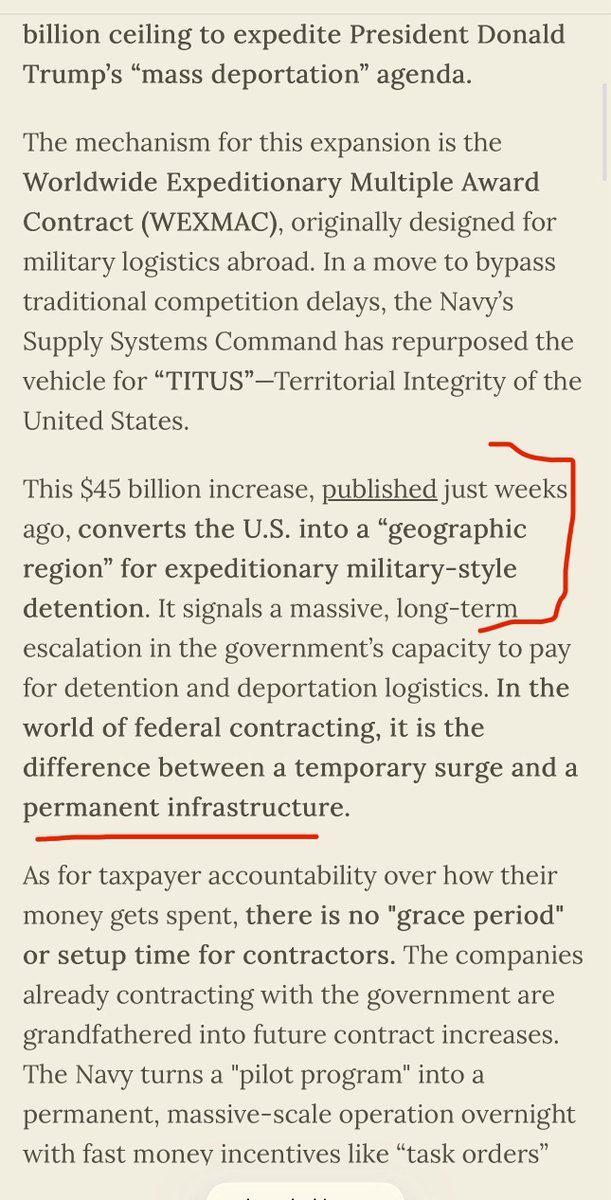
⚠️CDC & NY State is now recommending the IMMEDIATE testing & subtyping of all hospitalized flu A cases & unknown/suspected flu cases—in order to identify human bird flu. ➡️This implies CDC believes avian flu has now crossed into wide community transmission
nyc.gov/assets/doh/dow…

nyc.gov/assets/doh/dow…


2) Testing is good. But the widespread testing shows urgency.
“CDC is now recommending ordering subtyping for all influenza A-positive specimens collected from hospitalized individuals within 24 hours of hospital admission”
“CDC is now recommending ordering subtyping for all influenza A-positive specimens collected from hospitalized individuals within 24 hours of hospital admission”

3) And the memo says start testing with ANY SUSPECTED hospitalized cases of seasonal influenza as well. That’s quite broad.
The memo also doesn’t say NY residency required - thus are they implying anyone outside NYC (out-of-state too?) can also send specimens in to their lab.
The memo also doesn’t say NY residency required - thus are they implying anyone outside NYC (out-of-state too?) can also send specimens in to their lab.

4) this CDC advisory ACCELERATED SUBTYPING ON INFLUENZA A was issued by CDC before the recent Trump WH takeover. ➡️But the NY state memo goes above and beyond the CDC by recommending even subtype testing of even unknown hospital cases with unknown subtype.
cdc.gov/han/2025/han00…

cdc.gov/han/2025/han00…


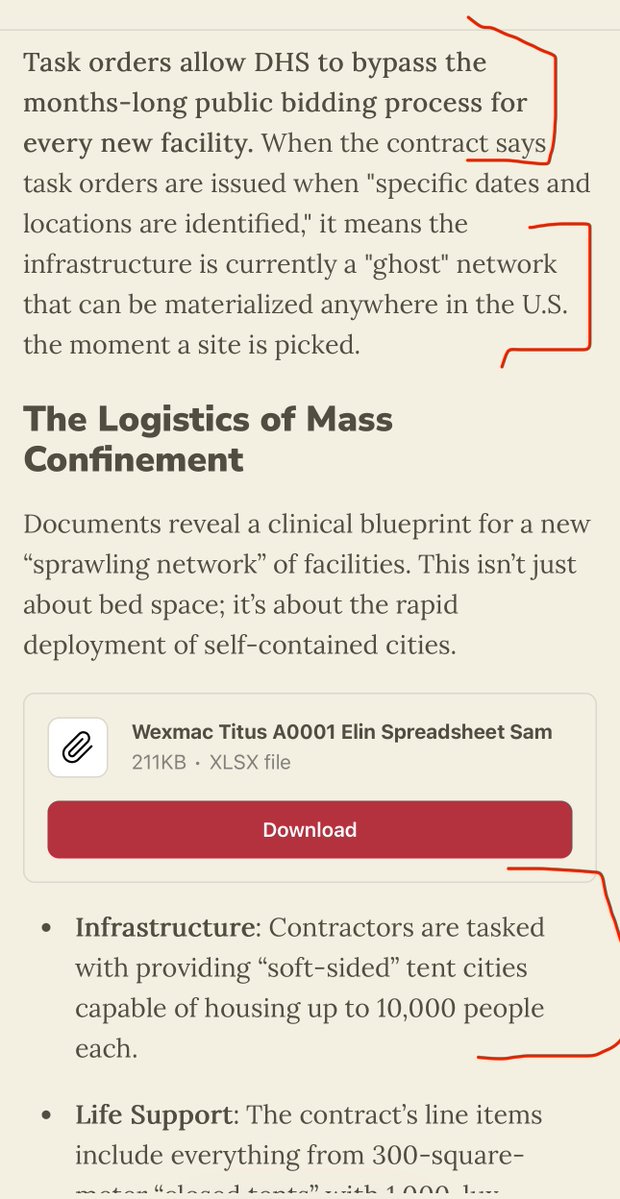
5) Furthermore “ Any hospitalized patients, especially those in an ICU, with suspected seasonal influenza or avian influenza A(H5) should be started on antiviral treatment with oseltamivir (TAMIFLU) as soon as possible **without waiting for the results of influenza testing**.”
6) AIRBORNE RISK: “if avian influenza A(H5) is suspected, place the individual in an airborne infection isolation room (AllR) with negative pressure, with standard, contact, and airborne precautions.” 

7) MASKING & HEPA—“If none is available, place a facemask on the individual and isolate in an examination room with the door closed. The individual should not be placed in any room where room exhaust is recirculated without high-efficiency particulate air (HEPA) filtration.” 

8) HUMAN TO HUMAN TRANSMISSION—Think about it—why would NY State issue the recommendations for 📌AIRBORNE TRANSMISSION and for 📌MASKING & 📌HEPA FILTRATION unless they specifically believe it is now human-to-human? Just reread this—it DOES NOT sound just animal contact anymore. 

9) Keep in mind that CDC pages are being deleted left and right. And they cannot issue new memos beyond Jan 20th. But do you know who can? NY and other states. ➡️I believe CDC wants to issue the update but can’t. Hence NY State issuing a broader warning to test & airborne risk.
https://twitter.com/drericding/status/1885495988963426460
10) If you don’t know, then get a cheap home test that deciphers Flu A, Flu B, vs COVID. If you identify Flu A, then you can try to ask for further testing from your state if it’s bird flu.
📍Stack these promo codes: “BF25” and “WELCOME5” for 25% off…
store.pharmalynk.com/products/cordx…
📍Stack these promo codes: “BF25” and “WELCOME5” for 25% off…
store.pharmalynk.com/products/cordx…

11) Fun fact - this PSA message to get the COVID and flu vaccines are not longer running under the Trump administration. Yet it can save people’s lives if they knew the power of booster shots.
https://twitter.com/drericding/status/1887692906766504181
12) Confirmed - household transmission of bird flu between cats and humans in two separate households. Also it was muzzled.
https://twitter.com/drericding/status/1887710100611125604
13) COMBO TEST OPTION #2: A lot of people are asking about Flu A tests… this FlowFlex Plus combo test is $1 more than the other one above, but it has 8% higher Sensitivity (92%) accuracy than the other test for Flu A, and 4% higher Sensitivity for Flu B.
If you identify Flu A, then you can try to ask for further testing from your state whether it’s bird flu or not.
👉Stack these promo codes: “BF25” and “WELCOME5” for 25% off…
store.pharmalynk.com/products/flowf…
If you identify Flu A, then you can try to ask for further testing from your state whether it’s bird flu or not.
👉Stack these promo codes: “BF25” and “WELCOME5” for 25% off…
store.pharmalynk.com/products/flowf…

• • •
Missing some Tweet in this thread? You can try to
force a refresh