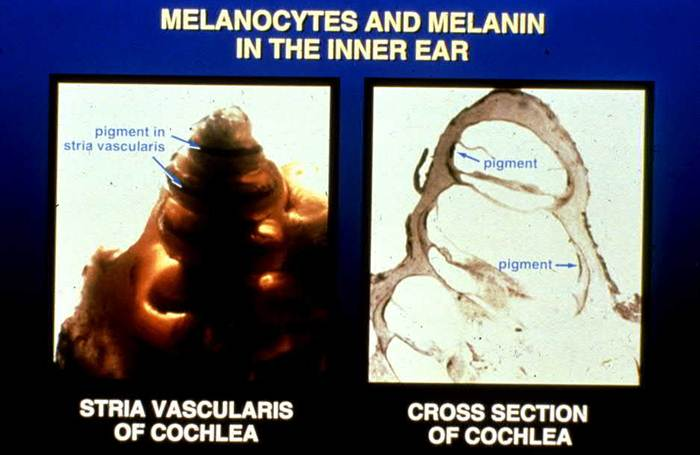
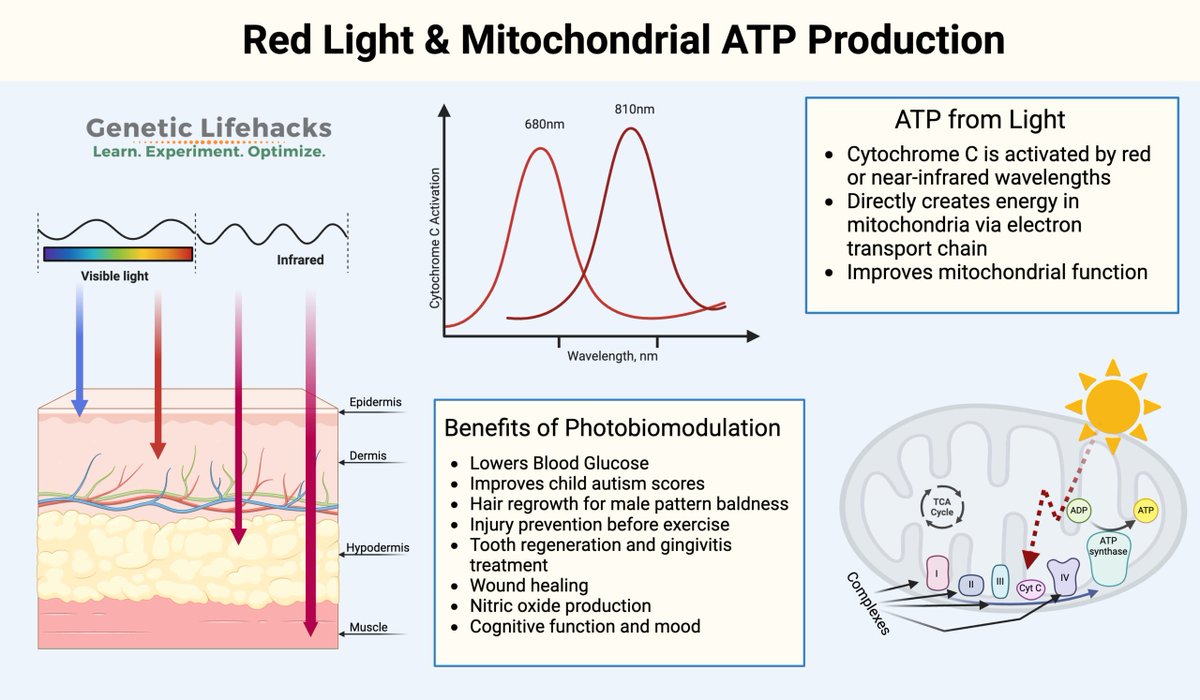
Red light from sunrise is essential for jumpstarting ATP production in your mitochondria.
Here's why you should never miss another sunrise for the rest of your life🧵
Here's why you should never miss another sunrise for the rest of your life🧵

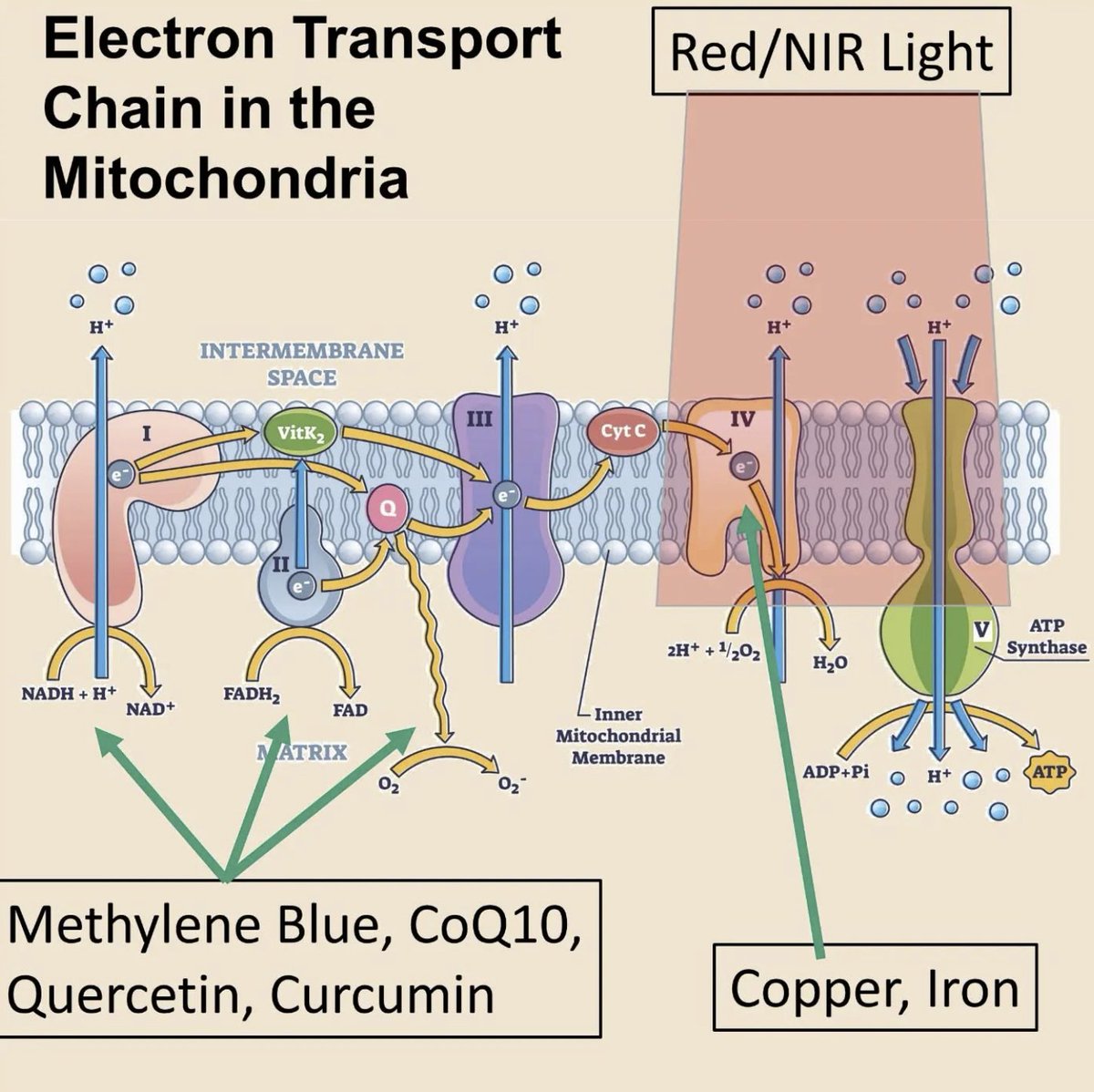
When Red/NIR Photons Interact With Your Mitochondria:
They're absorbed by cytochrome c oxidase's heme and copper centers.
This boosts CCO’s ability to convert oxygen into water (a key step in oxidative phosphorylation) →
Thereby accelerating electron transport, proton pumping, and ATP synthesis.
They're absorbed by cytochrome c oxidase's heme and copper centers.
This boosts CCO’s ability to convert oxygen into water (a key step in oxidative phosphorylation) →
Thereby accelerating electron transport, proton pumping, and ATP synthesis.
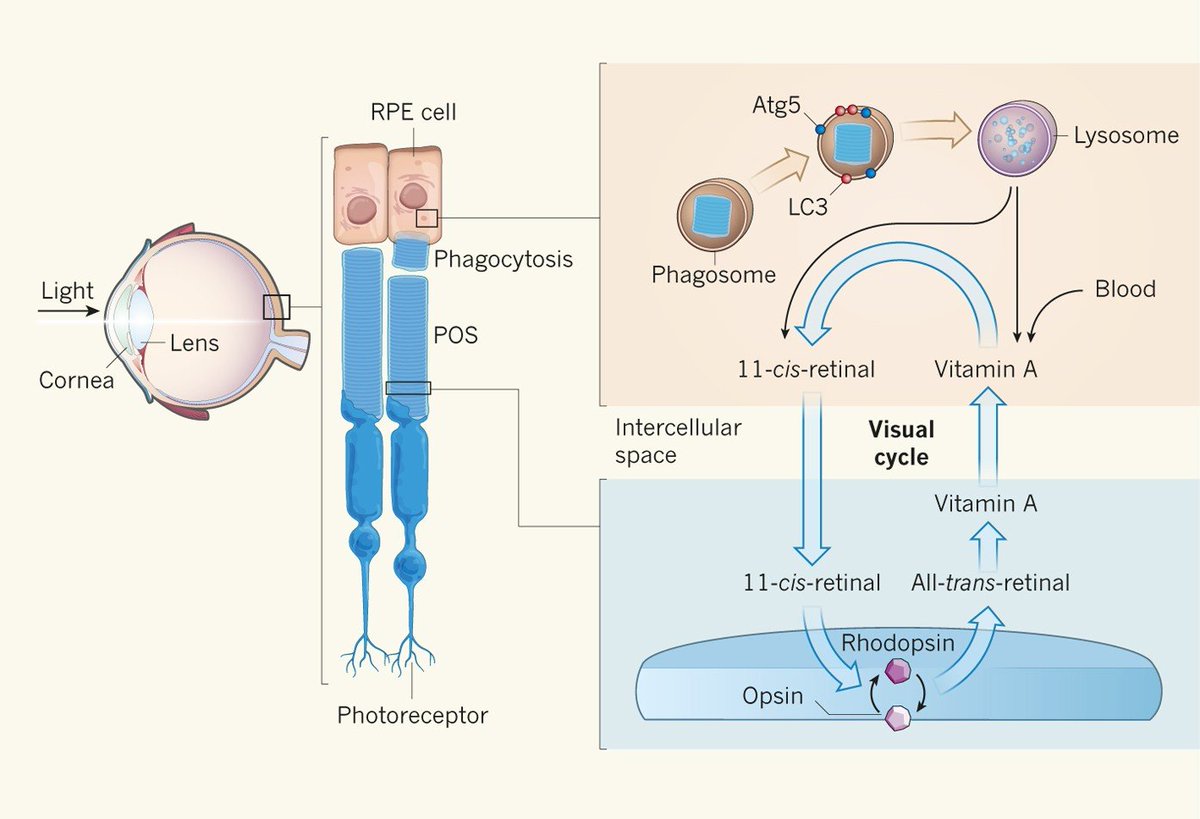
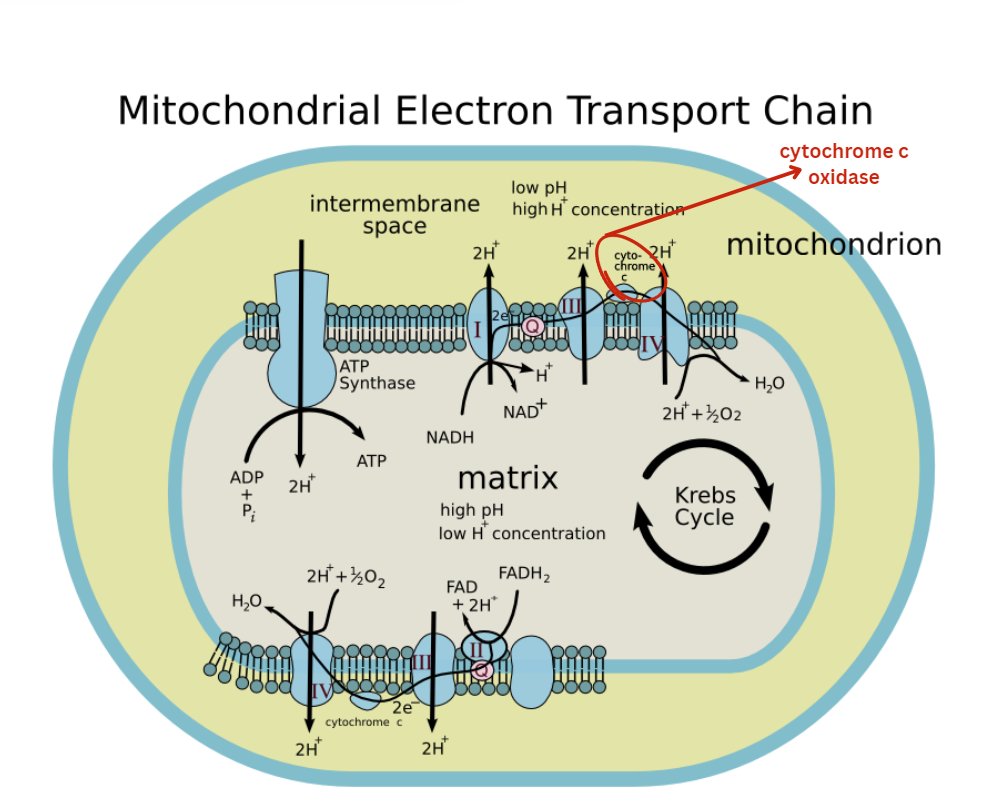
Before we get to the cool stuff... We need to address What is Cytochrome C Oxidase (CCO)?
CCO is a large transmembrane protein complex embedded in the inner mitochondrial membrane.
It is the terminal enzyme of the ETC, where it catalyzes the reduction of molecular oxygen (O2) to water (H2O).
This reaction is coupled to proton pumping across the membrane, creating the electrochemical gradient that drives ATP synthesis.
CCO is a large transmembrane protein complex embedded in the inner mitochondrial membrane.
It is the terminal enzyme of the ETC, where it catalyzes the reduction of molecular oxygen (O2) to water (H2O).
This reaction is coupled to proton pumping across the membrane, creating the electrochemical gradient that drives ATP synthesis.
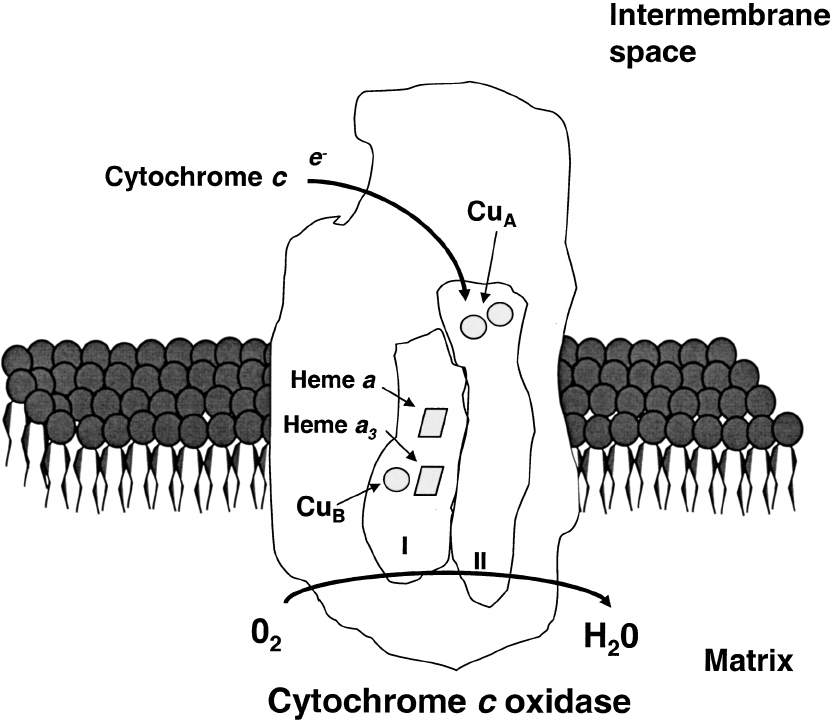
Cytochrome C oxidase contains four metal cofactors that act as chromophores for red/NIR light (600-900nm)
1. Heme A (subunit 1): absorbs at 605 nm
2. Heme A3 (Subunit 1): Absorbs at 655 nm, binds O2 at its Fe center.
3. CuA (subunit 2): absorption peaks at 620 nm (reduced) and 800–830 nm (oxidized), election entry site from cytochrome C
4. CuB (Subunit 1): absorbs at 760nm, paired with heme a3 in the binuclear center.
These metal centers enable CCO to act as a photoreceptor to sunrise light.
More on what a chromophore is here: x.com/Zenfrog4/statu…
This absorption excites their electrons, altering their redox states and enhancing CCO's enzymatic activity.
1. Heme A (subunit 1): absorbs at 605 nm
2. Heme A3 (Subunit 1): Absorbs at 655 nm, binds O2 at its Fe center.
3. CuA (subunit 2): absorption peaks at 620 nm (reduced) and 800–830 nm (oxidized), election entry site from cytochrome C
4. CuB (Subunit 1): absorbs at 760nm, paired with heme a3 in the binuclear center.
These metal centers enable CCO to act as a photoreceptor to sunrise light.
More on what a chromophore is here: x.com/Zenfrog4/statu…
This absorption excites their electrons, altering their redox states and enhancing CCO's enzymatic activity.
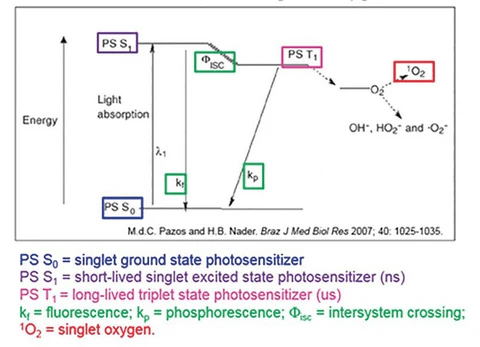
How Sunrise Generates ATP:
1. Normally, O2 exists in its "triplet" ground state, which has two unpaired electrons.
This triplet state is relatively inert because its unpaired electrons must pair with electrons from CCO's active site during reduction.
Luckily, molecular oxygen absorbs long-wavelength Red/ NIR radiation (in sunrise), which energizes its electrons and triggers a transition to its highly reactive "singlet" configuration.
Singlet oxygen has paired elections in only one orbital, making it more MUCH MORE REACTIVE, electrophilic, and as a result, more readily reduced to CCO.
More singlet oxygen due to red light also = upregulation of antioxidant enzymes like SOD
multiflora-herbs.com/blogs/news/ele…
frontiersin.org/journals/plant…
1. Normally, O2 exists in its "triplet" ground state, which has two unpaired electrons.
This triplet state is relatively inert because its unpaired electrons must pair with electrons from CCO's active site during reduction.
Luckily, molecular oxygen absorbs long-wavelength Red/ NIR radiation (in sunrise), which energizes its electrons and triggers a transition to its highly reactive "singlet" configuration.
Singlet oxygen has paired elections in only one orbital, making it more MUCH MORE REACTIVE, electrophilic, and as a result, more readily reduced to CCO.
More singlet oxygen due to red light also = upregulation of antioxidant enzymes like SOD
multiflora-herbs.com/blogs/news/ele…
frontiersin.org/journals/plant…
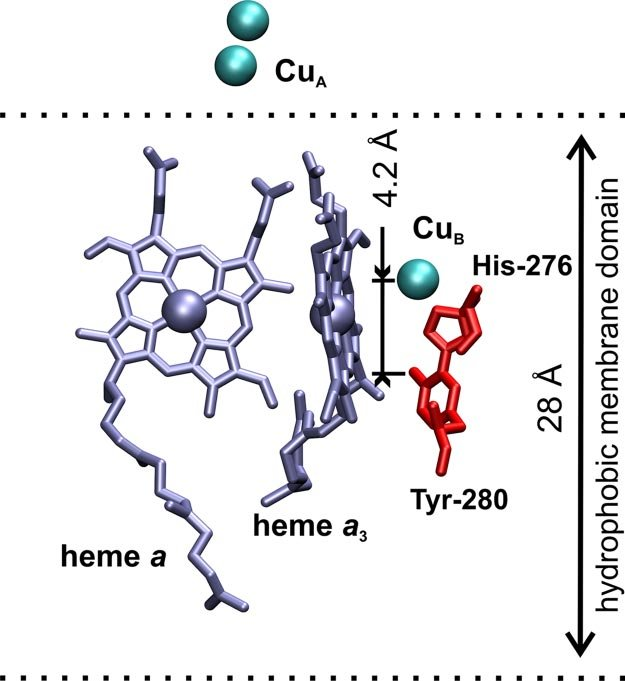
2. Red/ NIR photons excite electrons in heme a/a3 and CuA/CuB centers, altering their redox states.
This disrupts inhibitory nitric oxide (NO) binding to the a3-CuB center, restoring O2 affinity.
Oxygen gets activated, converting it from its triplet ground state of O2 into its reactive singlet state.
Singlet oxygen’s higher electrophilicity accelerates its reduction to water at the a3-CuB center.
This matters because singlet oxygen’s enhanced reactivity overcomes the kinetic barrier of triplet O₂’s unpaired electrons ->
Enabling efficient electron transfer and oxygen reduction at lower energy costs, and occurs via energy transfer from excited CCO chromophores

This disrupts inhibitory nitric oxide (NO) binding to the a3-CuB center, restoring O2 affinity.
Oxygen gets activated, converting it from its triplet ground state of O2 into its reactive singlet state.
Singlet oxygen’s higher electrophilicity accelerates its reduction to water at the a3-CuB center.
This matters because singlet oxygen’s enhanced reactivity overcomes the kinetic barrier of triplet O₂’s unpaired electrons ->
Enabling efficient electron transfer and oxygen reduction at lower energy costs, and occurs via energy transfer from excited CCO chromophores
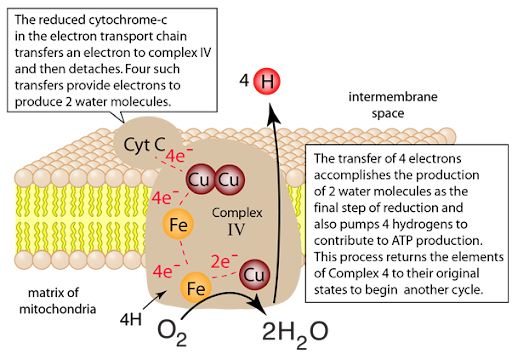
3. Light-enhanced CCO activity increases electron transfer from cytochrome c to O2, driving 4H+/electron across the inner mitochondrial membrane.
This amplifies the proton gradient (Δψm)
Once O2 binds to the binuclear center (heme a3-CuB), it undergoes sequential reduction.
- O2 accepts four electrons from cytochrome c via heme a -> heme a3 -> CuB.
- These reductions convert O2 into water (H2O), releasing energy that is used to pump protons across the inner mitochondrial membrane.
This amplifies the proton gradient (Δψm)
Once O2 binds to the binuclear center (heme a3-CuB), it undergoes sequential reduction.
- O2 accepts four electrons from cytochrome c via heme a -> heme a3 -> CuB.
- These reductions convert O2 into water (H2O), releasing energy that is used to pump protons across the inner mitochondrial membrane.
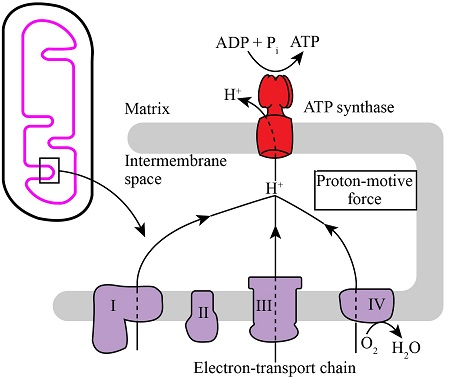
For every molecule of O2 reduced to water:
- Four protons (H+) are pumped from the mitochondrial matrix into the intermembrane space.
- This creates an electrochemical gradient across the membrane known as the proton motive force (PMF)
The PMF drives protons back into the matrix through ATP synthase, a rotary enzyme complex.
As protons flow down their gradient, ATP synthase couples this movement to phosphorylate ADP into ATP.
The PMF consists of:
- A chemical gradient (ΔpH): Higher H+ concentration in the intermembrane space than in the matrix
- An electrical gradient (Δψm): The intermembrane space becomes positively charged relative to the matrix.
- Four protons (H+) are pumped from the mitochondrial matrix into the intermembrane space.
- This creates an electrochemical gradient across the membrane known as the proton motive force (PMF)
The PMF drives protons back into the matrix through ATP synthase, a rotary enzyme complex.
As protons flow down their gradient, ATP synthase couples this movement to phosphorylate ADP into ATP.
The PMF consists of:
- A chemical gradient (ΔpH): Higher H+ concentration in the intermembrane space than in the matrix
- An electrical gradient (Δψm): The intermembrane space becomes positively charged relative to the matrix.
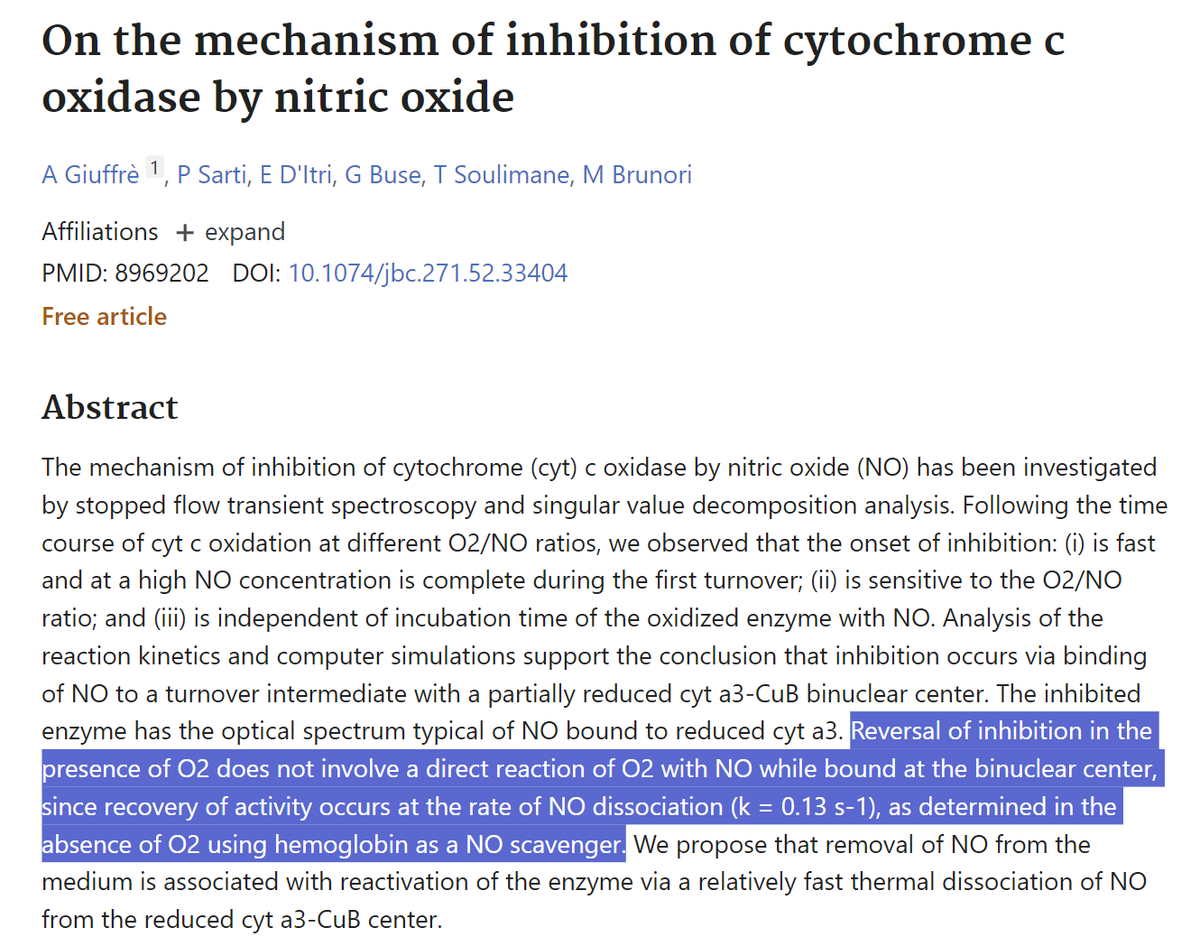
The Role of Nitric Oxide
Nitric Oxide (NO) is a signaling molecule that can inhibit CCO by binding to its a3-CuB center, competing with O2 for binding.
This inhibition slows down electron transport and reduces ATP production.
Red/NIR light photodissociates NO from CCO's active site.
CCO-NO + hν → CCO + NO
This restores CCO's activity by freeing up its active site for O2 binding.
Additionally.
-Reduced, NO levels improve mitochondrial respiration.
-NO dissociation enhances vasodilation by increasing its availability elsewhere in tissues.
4. Photodissociation of NO from CCO prevents competitive inhibition of O2 binding, improving respiratory efficiency (rate .13 s^-1 )
Nitric Oxide (NO) is a signaling molecule that can inhibit CCO by binding to its a3-CuB center, competing with O2 for binding.
This inhibition slows down electron transport and reduces ATP production.
Red/NIR light photodissociates NO from CCO's active site.
CCO-NO + hν → CCO + NO
This restores CCO's activity by freeing up its active site for O2 binding.
Additionally.
-Reduced, NO levels improve mitochondrial respiration.
-NO dissociation enhances vasodilation by increasing its availability elsewhere in tissues.
4. Photodissociation of NO from CCO prevents competitive inhibition of O2 binding, improving respiratory efficiency (rate .13 s^-1 )
What Makes Sunrise Unique?
Dawn sunlight peaks at 660 nm (red) and 850mm (NIR), aligning with CCO's absorption maxima.
frontiersin.org/journals/neuro…
And, as mentioned, morning light pulses generate transient singlet oxygen, which:
- Upregulates antioxidant enzymes (SOD, catalase) via Nrf2/ARE pathways.
- Activates circadian transcription factors (CLOCK/BMAL1) via ROS-mediated phosphorylation
- Circadian-driven ATP rhythms synchronize peripheral clocks, priming metabolism for daytime activity.
trainerize.me/articles/sunli…
researchgate.net/figure/Early-m…
Dawn sunlight peaks at 660 nm (red) and 850mm (NIR), aligning with CCO's absorption maxima.
frontiersin.org/journals/neuro…
And, as mentioned, morning light pulses generate transient singlet oxygen, which:
- Upregulates antioxidant enzymes (SOD, catalase) via Nrf2/ARE pathways.
- Activates circadian transcription factors (CLOCK/BMAL1) via ROS-mediated phosphorylation
- Circadian-driven ATP rhythms synchronize peripheral clocks, priming metabolism for daytime activity.
trainerize.me/articles/sunli…
researchgate.net/figure/Early-m…
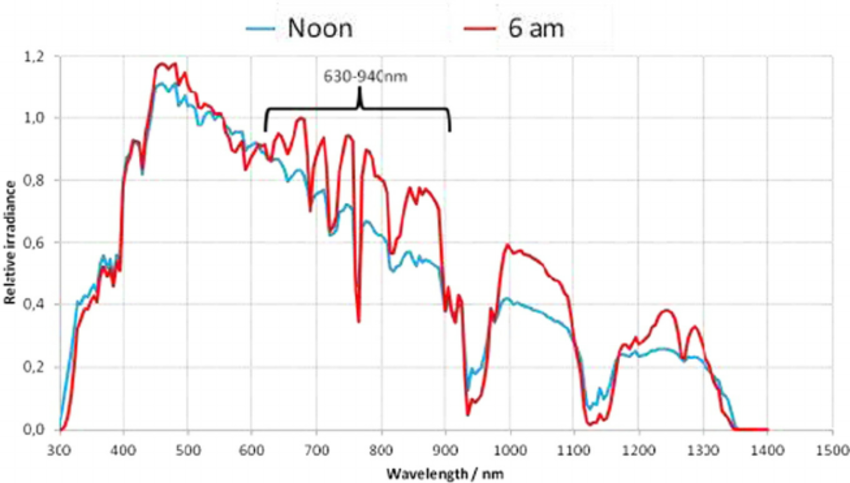
What's the Biological Impact of Morning Light Exposure?
In the short term, it speeds ATP synthesis significantly
And in the long run :
- Increased mitochondrial biogenesis (PGC-1a activation)
- Reduced oxidative Stress via glutathione upregulation
- Improved mood/ alertness from dopamine/serotonin surges
journals.physiology.org/doi/full/10.11…
nature.com/articles/s4159…
onlinelibrary.wiley.com/doi/10.1002/jb…
In the short term, it speeds ATP synthesis significantly
And in the long run :
- Increased mitochondrial biogenesis (PGC-1a activation)
- Reduced oxidative Stress via glutathione upregulation
- Improved mood/ alertness from dopamine/serotonin surges
journals.physiology.org/doi/full/10.11…
nature.com/articles/s4159…
onlinelibrary.wiley.com/doi/10.1002/jb…

In conclusion:
Sunrise photons, absorbed by the red/NIR light-accepting heme (Fe) and copper (Cu) centers of Cytochrome c Oxidase (CCO), enhance ATP production by increasing oxygen affinity, facilitating electron transport, and promoting a reactive singlet state.
CCO’s metal centers (heme A, heme A3, CuA, CuB) act as chromophores, absorbing 600–900 nm wavelengths to drive oxygen reduction and proton pumping.
This process is further supported by the photodissociation of inhibitory nitric oxide (NO) from CCO, improving respiratory efficiency.
In the long term, morning light exposure promotes mitochondrial biogenesis, reduces oxidative stress, and improves mood and alertness by synchronizing circadian rhythms...
Thereby optimizing cellular energy and function that artificial lighting or waking up late cannot replicate.
Sunrise photons, absorbed by the red/NIR light-accepting heme (Fe) and copper (Cu) centers of Cytochrome c Oxidase (CCO), enhance ATP production by increasing oxygen affinity, facilitating electron transport, and promoting a reactive singlet state.
CCO’s metal centers (heme A, heme A3, CuA, CuB) act as chromophores, absorbing 600–900 nm wavelengths to drive oxygen reduction and proton pumping.
This process is further supported by the photodissociation of inhibitory nitric oxide (NO) from CCO, improving respiratory efficiency.
In the long term, morning light exposure promotes mitochondrial biogenesis, reduces oxidative stress, and improves mood and alertness by synchronizing circadian rhythms...
Thereby optimizing cellular energy and function that artificial lighting or waking up late cannot replicate.

Thanks so much for reading!
You can find my other science breakdowns on my Substack:
That's all for now, have a great day.🐸🧙♂️🌠substack.com/@zenfrog4
You can find my other science breakdowns on my Substack:
That's all for now, have a great day.🐸🧙♂️🌠substack.com/@zenfrog4
• • •
Missing some Tweet in this thread? You can try to
force a refresh