New peer-reviewed study finds that the evidence base for pediatric gender medicine is weak: individual studies are inconsistent, and systematic reviews show no clear benefits. Guidelines that state or imply that medical transition is the standard of care aren't evidence-based. /1 

The study is not a systematic review (a limitation that the authors note), but it provides a comprehensive overview about what's known about the evidence base underpinning youth gender medicine. /2
link.springer.com/article/10.100…
link.springer.com/article/10.100…
Studies originating from gender clinics tend to focus on measuring the magnitude of the hypothesized psychological benefits. There is less focus on studying the risks and harms. This new publication provides a succinct summary of expected harms, along with the rationale. /3 

The authors illustrate how selective outcome reporting compromises even the best-known studies in the field, such as the study by Chen et al. This NIH-funded study tracked 19+ measures, but reported on only 4. The study also spun mixed/disappointing outcomes as highly positive./4 

The authors bring in an important bioethical angle, discussing the implications of recommending treatments to minors when the harms are expected to be significant but the benefits are at best uncertain. They observe:
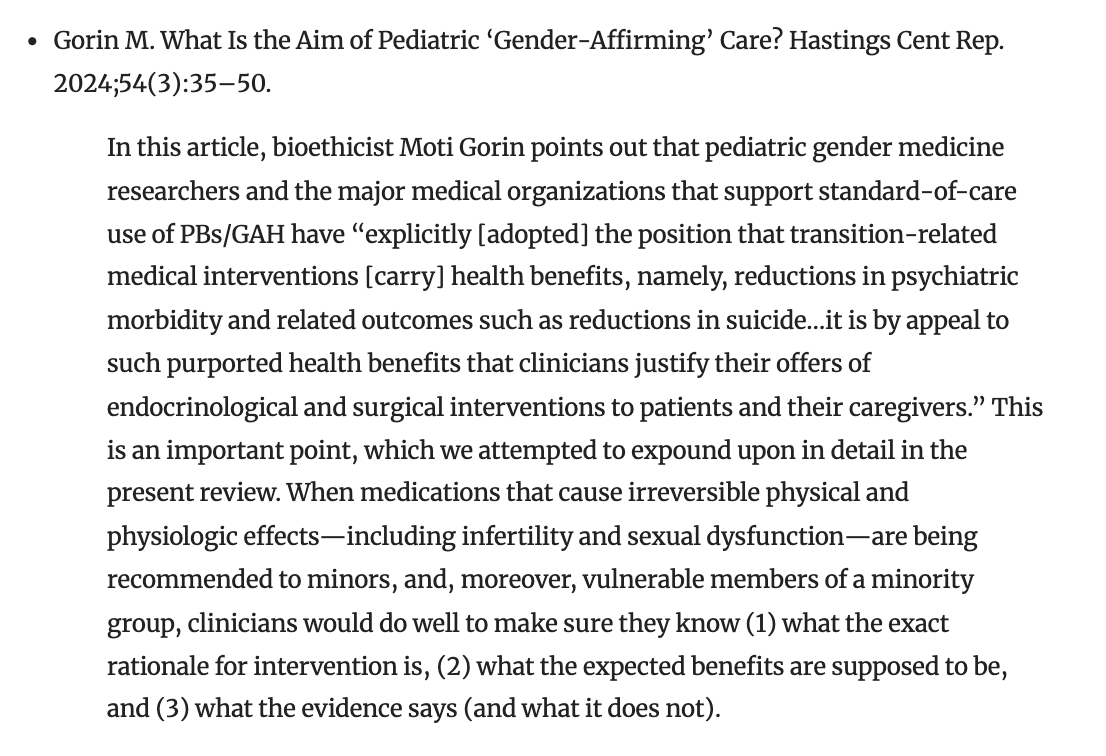
"When medications that cause irreversible physical and physiologic effects—including infertility and sexual dysfunction—are being recommended to minors, and, moreover, vulnerable members of a minority group, clinicians would do well to make sure they know (1) what the exact rationale for intervention is, (2) what the expected benefits are supposed to be, and (3) what the evidence says (and what it does not)." /5

"When medications that cause irreversible physical and physiologic effects—including infertility and sexual dysfunction—are being recommended to minors, and, moreover, vulnerable members of a minority group, clinicians would do well to make sure they know (1) what the exact rationale for intervention is, (2) what the expected benefits are supposed to be, and (3) what the evidence says (and what it does not)." /5

The new publication also discuses the Cass Review, which is a comprehensive review of pediatric gender medicine in the UK, with significant worldwide implications. Those uncertain about what the Cass Review is will find a succinct summary helpfully provided by the authors. /6 

The authors also provide a helpful explanation of "umbrella reviews," which are similar to systematic reviews (SR) but the unit of analysis is an SR rather than an individual study. This methodology was utilized in the HHS evidence review of the practice published May 1st. /7 

The authors conclude that there are serious risks to hormonal interventions for gender-distressed youth yet evidence of mental health benefit is weak. They emphasize the need to update guidelines in pediatric gender medicine to reflect the reality of the limited evidence. /8 

The full study, "Pediatric Gender Affirming Care is Not Evidence-based," is available at the link below:
link.springer.com/article/10.100…
link.springer.com/article/10.100…
• • •
Missing some Tweet in this thread? You can try to
force a refresh