💣You only need 6 documents to blow the lid off the scam! The video of lies is just a BONUS!
FDA rubber stamped an EUA, then handed Pfizer a license to kill—without ever verifying a shred of Pfizer’s data!
• 20Nov2020 EUA Memo
• 11Dec2020 EUA Letter
• 18May2021 BLA Clinical Review Memo
• 03Jun2021 First Committee Meeting Memo
• 13Aug2021 BIMO Review Memo
• 23Aug2021 BLA Approval Letter
FDA rubber stamped an EUA, then handed Pfizer a license to kill—without ever verifying a shred of Pfizer’s data!
• 20Nov2020 EUA Memo
• 11Dec2020 EUA Letter
• 18May2021 BLA Clinical Review Memo
• 03Jun2021 First Committee Meeting Memo
• 13Aug2021 BIMO Review Memo
• 23Aug2021 BLA Approval Letter
20Nov2020 EUA Memo
Claim: "6 site inspections did not reveal problems impacting the data submitted in support of this EUA."
Reality: The FDA lied! They did go to 6 sites, but no problems were revealed because they NEVER looked; they DID NOT verify the data Pfizer submitted!
Next slide, please.
Claim: "6 site inspections did not reveal problems impacting the data submitted in support of this EUA."
Reality: The FDA lied! They did go to 6 sites, but no problems were revealed because they NEVER looked; they DID NOT verify the data Pfizer submitted!
Next slide, please.

11Dec2020 EUA Letter
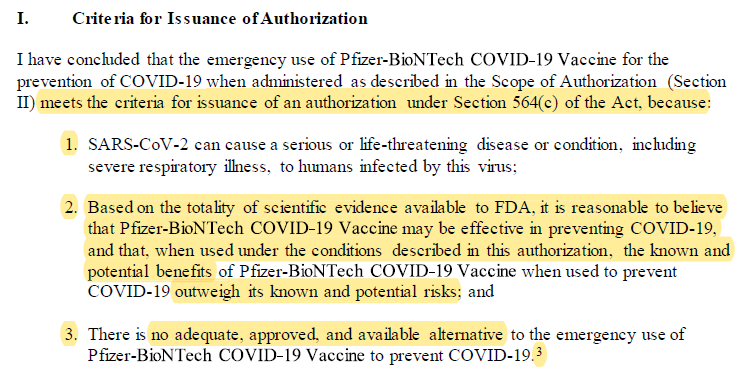
Claim: Criteria Met for Issuance of Authorization
**If you believe the load under Section 564(c) of the Act highlighted below.**
Reality: The EUA law DOES give the FDA more flexibility during review. What it DOES NOT do is allow FDA to lie about that discretion! There's a legal term for that, I'm sure.
Next slide, please.
Claim: Criteria Met for Issuance of Authorization
**If you believe the load under Section 564(c) of the Act highlighted below.**
Reality: The EUA law DOES give the FDA more flexibility during review. What it DOES NOT do is allow FDA to lie about that discretion! There's a legal term for that, I'm sure.
Next slide, please.
18May2021 BLA Clinical Review Memo
Claim: Carry On!
⚠️Pg. 5 “The clinical information was not well organized and a summary document would be helpful... the clinical team thought they will likely have a safety data information request.”
Reality: Even the clinical reviewers were unsure they had what they needed—they hinted they’d have to circle back to Pfizer to actually get clarity.
**Spoiler: they didn’t before approval!
Next slide, please.
Claim: Carry On!
⚠️Pg. 5 “The clinical information was not well organized and a summary document would be helpful... the clinical team thought they will likely have a safety data information request.”
Reality: Even the clinical reviewers were unsure they had what they needed—they hinted they’d have to circle back to Pfizer to actually get clarity.
**Spoiler: they didn’t before approval!
Next slide, please.
18May2021 BLA Clinical Review Memo Cont.
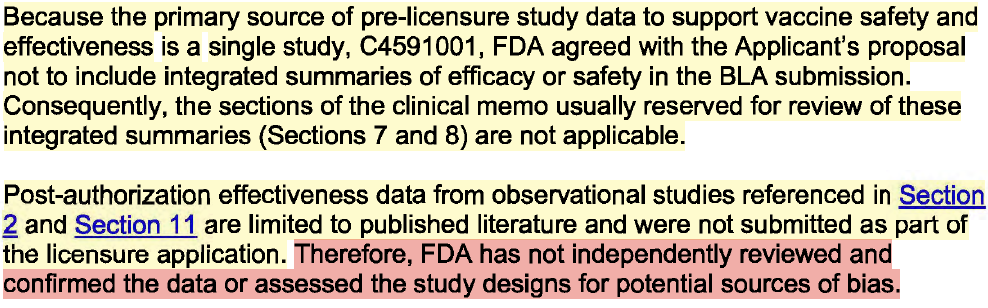
⚠️Pg. 19 "Because the primary source of pre-licensure study data to support vaccine safety and effectiveness is a single study, C4591001, FDA agreed with the Applicant’s proposal to include integrated summaries... The sections of the clinical memo usually reserved for review of these integrated summaries were not generated.”
Reality: The FDA did not verify or analyze key data inputs that were used to support the vaccine's safety and efficacy narrative!
"Therefore, FDA has not independently reviewed and confirmed the data or assessed the study designs for potential sources of bias."
Reality: WTAF!
No bias assessment and they’re admitting that part of the BLA application rests on unchecked claims from publications rather than validated trial datasets!
It gets worse, guys! Next slide, please.
⚠️Pg. 19 "Because the primary source of pre-licensure study data to support vaccine safety and effectiveness is a single study, C4591001, FDA agreed with the Applicant’s proposal to include integrated summaries... The sections of the clinical memo usually reserved for review of these integrated summaries were not generated.”
Reality: The FDA did not verify or analyze key data inputs that were used to support the vaccine's safety and efficacy narrative!
"Therefore, FDA has not independently reviewed and confirmed the data or assessed the study designs for potential sources of bias."
Reality: WTAF!
No bias assessment and they’re admitting that part of the BLA application rests on unchecked claims from publications rather than validated trial datasets!
It gets worse, guys! Next slide, please.
18May2021 BLA Clinical Review Memo Cont.
⚠️Pg. 15 “Bioresearch Monitoring inspections of nine clinical sites in study C4591001 did not identify deficiencies that would affect the integrity of the clinical data submitted in this BLA.”
Reality: This sounds reassuring until...
Next slide, please.
⚠️Pg. 15 “Bioresearch Monitoring inspections of nine clinical sites in study C4591001 did not identify deficiencies that would affect the integrity of the clinical data submitted in this BLA.”
Reality: This sounds reassuring until...
Next slide, please.

03Jun2021 First Committee Meeting Memo
This is just indefensible! The whole statement...WOW!
Claim 1: "10 study sites were already conducted under the IND and EUA..."
Reality: 10? They sure? Hang tight....
Claim 2: “The BIMO team stated that the inspections that occurred under the IND and EUA did not inspect data integrity because there wasn't anything to inspect at the time..”
We will come back to this in the next slide, please.
This is just indefensible! The whole statement...WOW!
Claim 1: "10 study sites were already conducted under the IND and EUA..."
Reality: 10? They sure? Hang tight....
Claim 2: “The BIMO team stated that the inspections that occurred under the IND and EUA did not inspect data integrity because there wasn't anything to inspect at the time..”
We will come back to this in the next slide, please.

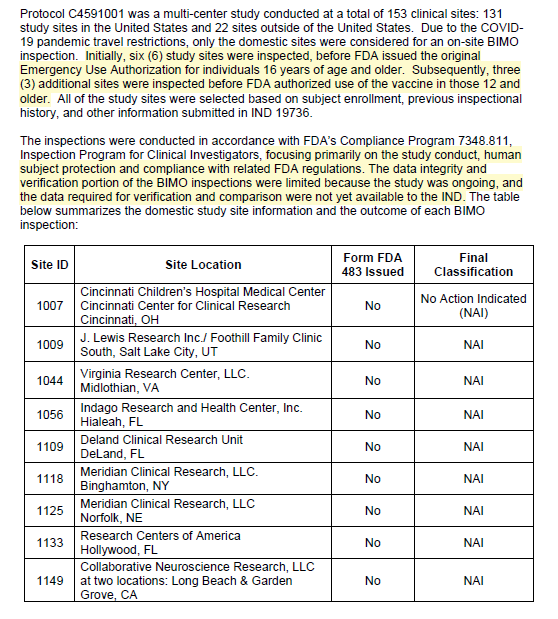
13Aug2021 BIMO Review Memo
Claim 1: The 03Jun2021 Committee Memo said FDA inspected 10 sites, but this memo says only 9 sites were inspected for the BLA.
Reality:
They can’t even keep their story straight. Did one site vanish? Are they hiding it? This was never about actual oversight. This was about creating the illusion of it.
And these numbers matter because each site represents hundreds of human beings whose data the FDA claimed to have reviewed.
Claim 2: “The data integrity and verification portion of the BIMO inspections were limited because the study was ongoing, and the data required for verification and comparison were not yet available to the IND.”
Reality: Let’s be blunt! FDA walked into 9 sites, then turned around and walked tf back out!
Then they lied to the world. Lied to the media. Lied to Congress. Lied to the courts. They told us everything had been rigorously inspected and found to be in order. They told us it was safe and effective because that's what Pfizer told them.
Those 9 sites collectively enrolled nearly 4,000 participant! What if they had just done their job?
What if they had looked even once at what was happening at those sites? My sites. Maybe Maddie de Garay (Site 1007) wouldn’t be in a wheelchair today. Maybe thousands more would have been spared harm.
But they didn’t. Final slide, please.
Claim 1: The 03Jun2021 Committee Memo said FDA inspected 10 sites, but this memo says only 9 sites were inspected for the BLA.
Reality:
They can’t even keep their story straight. Did one site vanish? Are they hiding it? This was never about actual oversight. This was about creating the illusion of it.
And these numbers matter because each site represents hundreds of human beings whose data the FDA claimed to have reviewed.
Claim 2: “The data integrity and verification portion of the BIMO inspections were limited because the study was ongoing, and the data required for verification and comparison were not yet available to the IND.”
Reality: Let’s be blunt! FDA walked into 9 sites, then turned around and walked tf back out!
Then they lied to the world. Lied to the media. Lied to Congress. Lied to the courts. They told us everything had been rigorously inspected and found to be in order. They told us it was safe and effective because that's what Pfizer told them.
Those 9 sites collectively enrolled nearly 4,000 participant! What if they had just done their job?
What if they had looked even once at what was happening at those sites? My sites. Maybe Maddie de Garay (Site 1007) wouldn’t be in a wheelchair today. Maybe thousands more would have been spared harm.
But they didn’t. Final slide, please.
23Aug2021 BLA Approval Letter
The FDA approved and promoted a vaccine based on clinical data it never verified, then misled the public about the rigor of its review. Internal documentation proves that:
The data verification never occurred.
Inspectors admitted there was “nothing to inspect.”
The data from thousands of trial participants was used to justify licensure without basic scrutiny.
The list goes on...
Meanwhile, public-facing statements told Americans and global regulators the exact opposite.
This constitutes regulatory fraud and demands immediate oversight, accountability, and corrective legislative action.
The end.
...unless you want me to tell you how Moderna did it-@SecKennedy
The FDA approved and promoted a vaccine based on clinical data it never verified, then misled the public about the rigor of its review. Internal documentation proves that:
The data verification never occurred.
Inspectors admitted there was “nothing to inspect.”
The data from thousands of trial participants was used to justify licensure without basic scrutiny.
The list goes on...
Meanwhile, public-facing statements told Americans and global regulators the exact opposite.
This constitutes regulatory fraud and demands immediate oversight, accountability, and corrective legislative action.
The end.
...unless you want me to tell you how Moderna did it-@SecKennedy

• • •
Missing some Tweet in this thread? You can try to
force a refresh








