People have been asking about the SARSCoV2 (COVID19) vaccine outlook so I'll summarize as best as I can. But overall, the current situation is chaotic and in constant flux, and if FDA is trying to apply consistent criteria then they haven't shown it yet
Right now there are 4 vaccines from 3 companies covering 2 technologies with FDA approval in some form or other. The approvals are for different age ranges and risk categories. In addition each August or September new formulations have to be reapproved, kind of like influenza.
The vax are Comirnaty (Pfizer), Spikevax (Moderna), mNEXSPIKE (Moderna), and Novaxovid (Novavax). The first 3 are RNA, the last is protein. HHS.gov shows who qualifies for each, but hasn't been updated with mNEXSPIKE. Must be the DOGE cuts hhs.gov/coronavirus/co… 

mNEXSPIKE differs from the other 3 in including only sequences for the RBD (receptor binding domain) of SARSCoV2 spike. The inclusion of only the RBD allows a lower RNA amount, which helps with side effects (a tacit admission that the original dose had noticeable effects)
mNEXSPIKE was approved in July for anyone ≥65yo and those ≥12yo at risk for severe COVID19, similar indication as for Novavax in May. Okay so that seems somewhat consistent, the first two full approvals under Makary and Prasad have the same indication
fda.gov/vaccines-blood…
fda.gov/vaccines-blood…
The FDA then (just a few days after mNEXSPIKE) fully approved the older SpikeVax formula for children 6 months to 11 years old at risk for severe COVID19. That moves that indication from EUA status to regular approval, the first for kids under 12.
reuters.com/business/healt…
reuters.com/business/healt…
But regular approval of SpikeVax for at-risk kids ≥6mo meant dropping the EUA approval of SpikeVax for low-risk kids ≥6mo, leaving Pfizer's EUA approval for all kids 6mo and older as the only option.
And that looks likely to go away now for kids <5yo
theguardian.com/us-news/2025/a…
And that looks likely to go away now for kids <5yo
theguardian.com/us-news/2025/a…
So the HHS website is probably going to end up looking like:
SpikeVax: everyone ≥12y (reg), higher-risk ≥6m (reg)
Comirnaty: everyone ≥12y (reg), everyone ≥5y (EUA)
mNEXSPIKE, Novavax: everyone ≥65y (reg), higher-risk ≥12y (reg)
Make sense? I didn't think so either.
SpikeVax: everyone ≥12y (reg), higher-risk ≥6m (reg)
Comirnaty: everyone ≥12y (reg), everyone ≥5y (EUA)
mNEXSPIKE, Novavax: everyone ≥65y (reg), higher-risk ≥12y (reg)
Make sense? I didn't think so either.
The broad indications for SpikeVax and Comirnaty is likely because it's hard to undo a regular approval, but it means the milder vaxxes (mNEXSPIKE, Novavax) are the only ones restricted to higher-risk. And there's an odd retention of ≥5y EUA for Pfizer if I infer correctly.
That's FDA; actual current CDC recommendations are broader: "Where the parent presents with a desire for their child to be vaccinated, children 6mo and older may receive COVID-19 vaccination, informed by the clinical judgment of a healthcare provider…"
cdc.gov/vaccines/hcp/i…
cdc.gov/vaccines/hcp/i…

The AAP is also very clear they recommend annual COVID19 shots to all children ≥6mo old
The headline is pretty sassy: "The American Academy of Pediatrics Releases Its Own Evidence-Based Immunization Schedule"
aap.org/en/news-room/n…
The headline is pretty sassy: "The American Academy of Pediatrics Releases Its Own Evidence-Based Immunization Schedule"
aap.org/en/news-room/n…
Anyway adding to uncertainty is that FDA has been slow to approve the 2025-2026 formulations. They had recommended a JN.1 lineage, preferably LP.8.1, and all 3 manufacturers made LP.8.1 shots. Pfizer and Moderna have made it past the EU's CHMP already
msn.com/en-us/health/o…
msn.com/en-us/health/o…
Novavax has filed for LP.8.1 in Japan, not sure if they will do LP.8.1 or parental JN.1 in the US (they had shown LP.8.1 boosters weren't better and might even be overspecialized to LP.8.1 vs other JN.1 substrains, below from
fda.gov/advisory-commi…
fda.gov/advisory-commi…
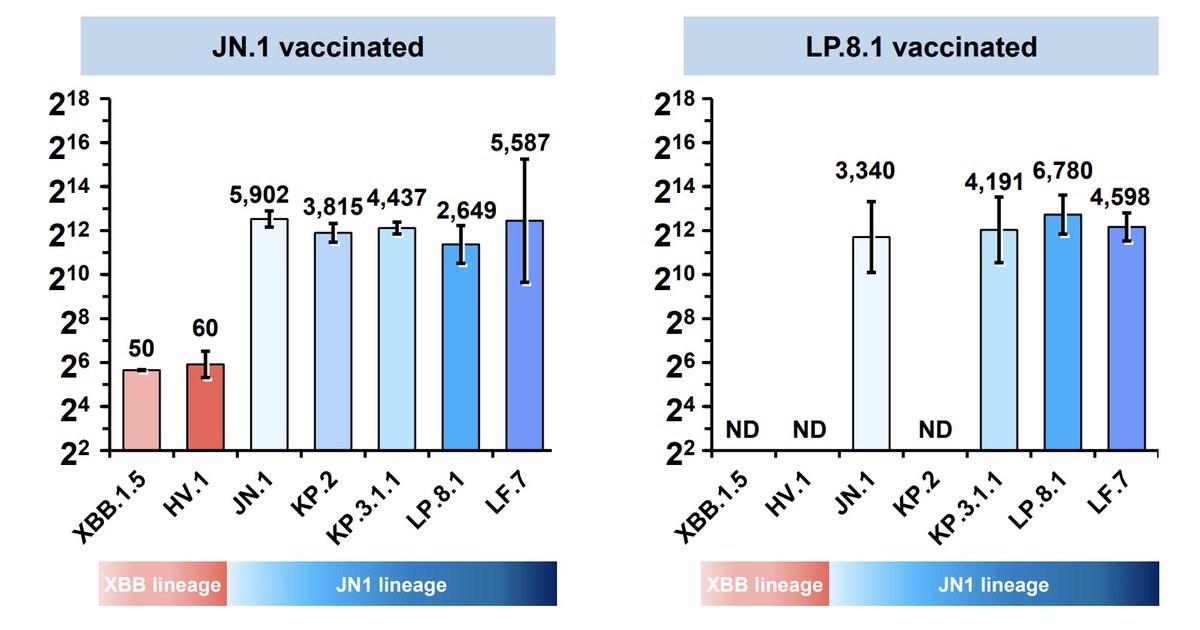
In any case, kids <2yo are at higher risk of complications than older kids, because their next SARSCoV2 infection is more likely to be their first, and thus they have no preexisting active immunity from the myriad cardiovascular, pulmonary, and neurological issues that can arise.
Thus it's terrible medical practice for FDA to only officially approve COVID vaccines for kids ≤5mo if they are at "higher risk". That age is at especially higher risk!
Prasad and Makary attempted to justify the new FDA policies in a NEJM opinion piece by saying that SARSCoV2 vaccines are only recommended in the EU for older adults and at-risk people of other ages. However this argument is misinformed or disingenuous.
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
The EMA approves the vaccines for everyone ≥6mo, and public health agencies then actively recommend it for those at risk. So it's more like everyone can get it, and we especially recommend it for those at risk. That's different from the drug not being approved for low-risk!
In the EU you can just get it, no questions asked. Here in the US you have to go through the additional hoop of asserting your child is at-risk. But if you needed any help convincing your doctor, you can choose from this official list published in that NEJM piece above 
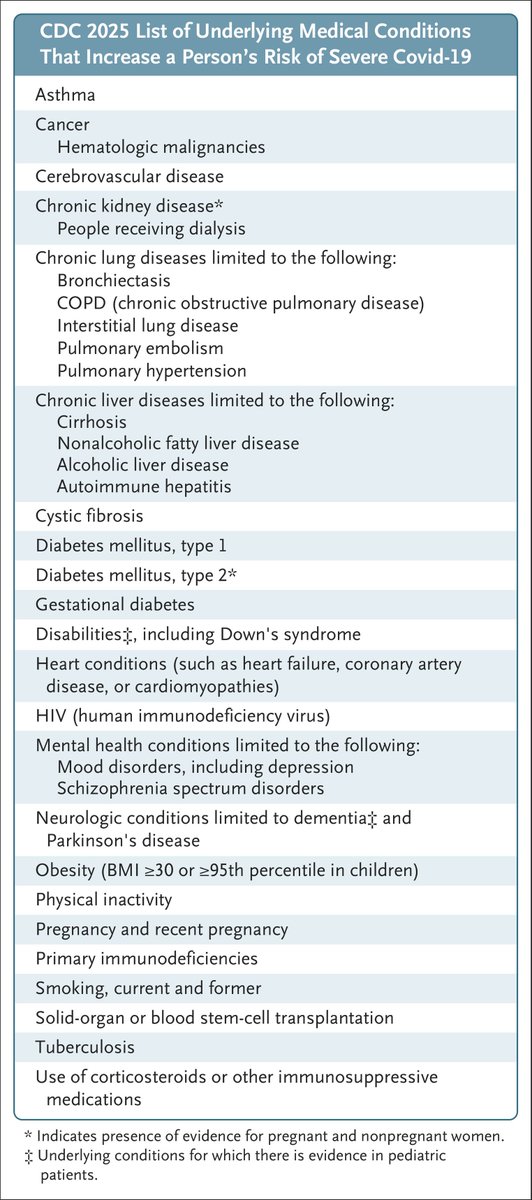
If you want to see the EU approvals, here they are. Note no wording about risk groups, just simple ages approved for each vaccine.
So yes the FDA is making it more complicated than necessary, and not saying the truth about it.
ema.europa.eu/en/human-regul…
So yes the FDA is making it more complicated than necessary, and not saying the truth about it.
ema.europa.eu/en/human-regul…
Oh I had forgotten that humans are supposed to be useless at summarizing publicly available information now that we have AI. Let's see if Grok can do better.
@grok can you summarize the current status of COVID-19 vaccines in the US?
@grok can you summarize the current status of COVID-19 vaccines in the US?
Good article on the AAP recommendations, which I forgot to mention just came out today
arstechnica.com/health/2025/08…
arstechnica.com/health/2025/08…
Pediatricians are the most chill docs; it takes a lot to get them into sassy mode. Yet FDA managed to do that.
• • •
Missing some Tweet in this thread? You can try to
force a refresh









