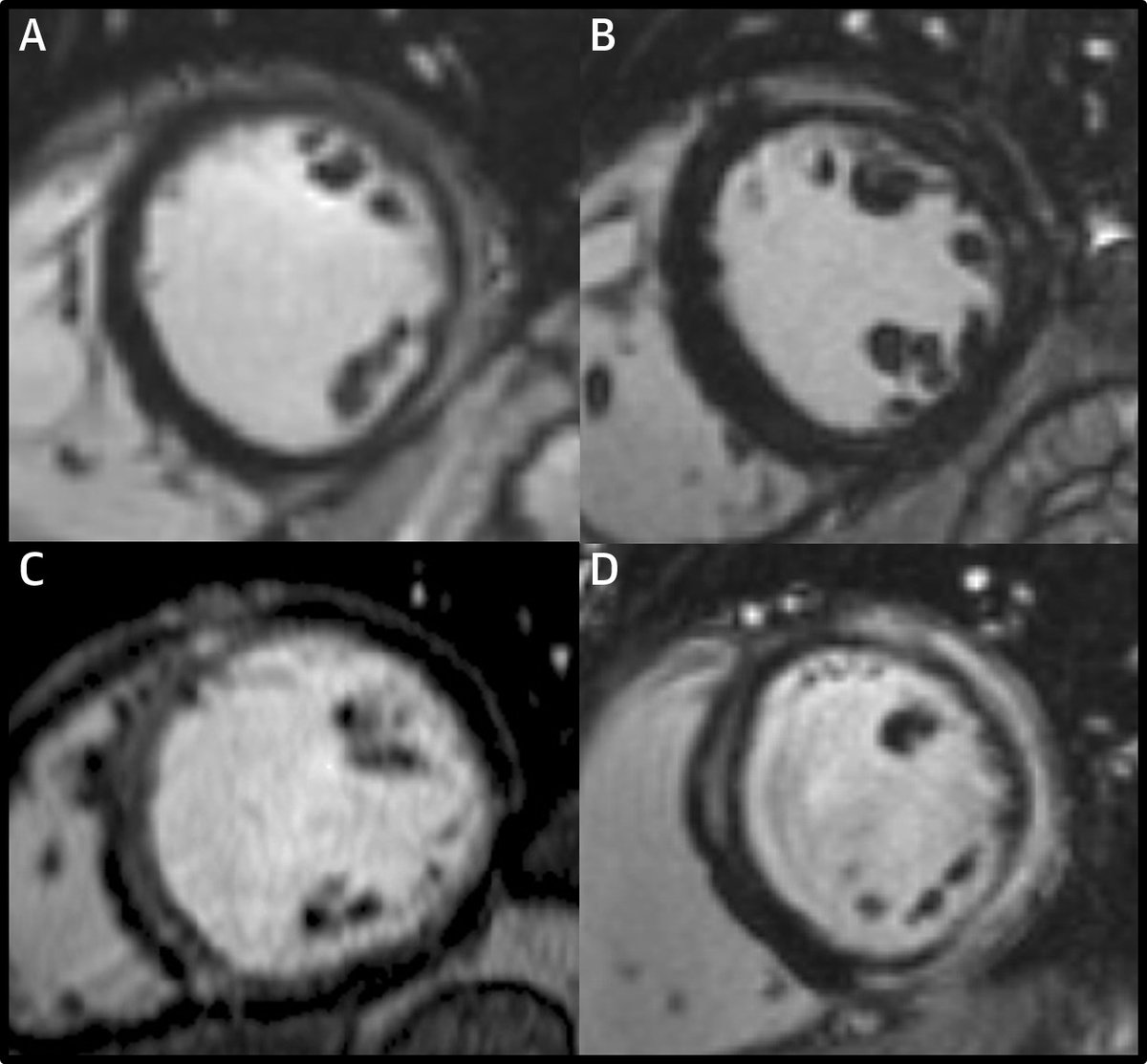
With all the discussion about viability in the past few days, I would like to share how I interpret and report viability on CMR. I first look for LGE. Rarely, there’s no LGE and it’s all viable or more likely, a non-ischemic cardiomyopathy. #WhyCMR 1/18
When I see LGE, I confirm it’s in an ischemic pattern – subendocardial or transmural, and limited to a coronary territory, i.e., an MI. If not, it's again a non-ischemic cardiomyopathy and not a viability issue anymore. 2/18
Next, I try to identify how many and where the MIs are. For this, I look at the extent and locations of ischemic LGE and decide which of the 17 LV segments are likely to be supplied by each of the coronary arteries. 3/18
This paper has data that I find useful to know which coronary artery is responsible for an MI involving a particular segment or a combination of segments: imaging.onlinejacc.org/content/1/3/282
For instance, LGE involving the basal anterolateral and inferolateral segments cannot be anything but an LCx MI but LGE involving the basal inferolateral and inferior segments can also be an RCA MI. 5/18 

I also look at the coronary angiogram to know how large the coronaries are, which coronaries supply which LV segments, whether the LAD wraps around the apical LV, etc. 6/18
Angiograms are always available because the two pre-requisites for requesting a viability study are: severe CAD in 1 or more coronary arteries and LV dysfunction. 7/18
Integrating all this information, I know how many MIs there are, which coronary territories they belong to, and I allocate the 17 LV segments between the main coronary arteries. 8/18
Next, I score the LGE in a semiquantitative way on a 17-segment basis based on the area of LGE, using a 5-point scale: 0 = no LGE; 1 = 1% to 25%; 2 = 26% to 50%; 3 = 51% to 75%; 4 = 76% to 100%.
One key point is NOT to look at transmurality at any single location (transmural thickness of LGE) but to consider each segment as a volume and visually estimate the amount of LGE within the volume of the segment (each segment = 1/17 of the LV mass). 10/18
I then estimate the extent of LGE within each territory by adding the segmental scores weighted by the mid-point of the range of LGE = 12.5% for 1 (midpoint of 1-25%), 37.5% for 2 (midpoint of 26-50%), etc. and dividing by the total number of segments in the territory. 11/18
Thus, I get an LGE extent in % for each of the coronary territories. 100 minus LGE extent in % gives me the viability in each territory, which I report in multiples of 5%. So, a patient may have 65% viability in the LAD, 30% in the RCA, and 100% in the LCx territories. 12/18
Viability is not a dichotomous entity. The extent of viability and locations of viability should be integrated with other factors such as quality of target vessels, surgical risk, presence of hibernating myocardium, etc. to decide whether to revascularize and how. 13/18
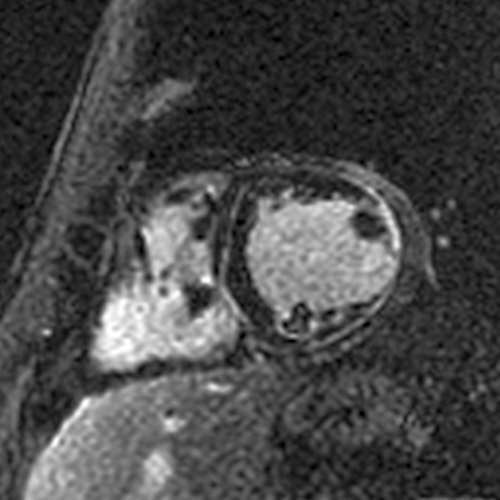
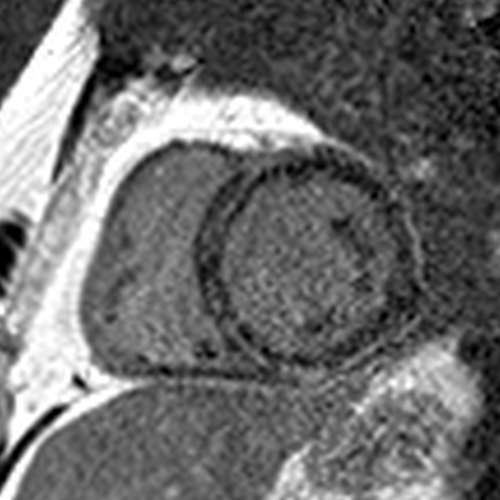
I assess the presence of hibernating myocardium by comparing LGE extent in a coronary territory to the wall motion and looking for discordance. The coronary supplying this territory should also have severe disease. 14/18
For instance, if there is 0-30% LGE with severe hypokinesis or akinesis (= dysfunction beyond what you would expect from the LGE), there is hibernating myocardium. If there is no or mild hypokinesis, there is no hibernating myocardium. 15/18
Hibernating myocardium = viable myocardium that’s hypocontractile due to severe ischemia. It has the potential for improvement in contractility, and some data suggest that hibernating myocardium is prognostically worse than ischemic-but-not-hibernating myocardium. 16/18
The summary of my typical viability CMR report looks something like this:
Ischemic cardiomyopathy, LVEF 28%, MIs in the LAD and RCA territories, 65% viability with hibernating myocardium in the LAD territory, 100% viability without hibernating myocardium in the LCx territory, and 30% viability without hibernating myocardium in the RCA territory. 18/18
• • •
Missing some Tweet in this thread? You can try to
force a refresh