How do you make nuclear pores away from the nucleus?
Our paper online today bit.ly/AL-NPC
@embl @a_schw @SchwabYannick @CellCellPress #development #Nuclearpores #microscopy
https://t.co/f4Xq1Vsk31
1/11 [a thread]
Our paper online today bit.ly/AL-NPC
@embl @a_schw @SchwabYannick @CellCellPress #development #Nuclearpores #microscopy
https://t.co/f4Xq1Vsk31
1/11 [a thread]
Background: Mothers stockpile nuclear pores (NPCs) in the cytoplasm of their eggs to enable embryonic cell cycles that are too fast for conventional NPC assembly (bit.ly/ALembryo). These are arranged in stacked ER sheets called Annulate Lamellae (AL, see 4/11).
2/11
2/11
These are Drosophila egg chambers; where the growing oocyte is transcriptionally silent, while so-called nurse cells produce the material for its development and transport it to the front. We wanted to see if this includes AL and whether they accumulate over time.
3/11
3/11

This is how a part of the ooplasm, including a rendered Annulate Lamellum looks like. The parallel stacked ER sheets containing NPCs are embedded within the ER in an elaborate membrane topology.
4/11
4/11
That oocytes contain AL was known since the '50s but to see if they actively accumulate, we first quantified them over oogenesis and saw local accumulation in the oocyte during development (red arrowheads).
5/11
5/11

With more NPC proteins marked, we saw not just AL (white), but also large spherical granules made up of specific subsets of NPC components. These granules had a very distinct spatial and temporal distribution suggesting their progression into AL.
6/11
6/11

We hypothesized that these granules might be precursors for AL. We found that one type of granule containing the Nup358 protein was actively transported from nurse cells to the oocyte along microtubules. There they interacted with other NPC granules to form AL.
7/11
7/11
So how do these granules look like? Like little droplets, diving into each other. This is how they look in fluorescence confocal microscopy…
8/11
8/11
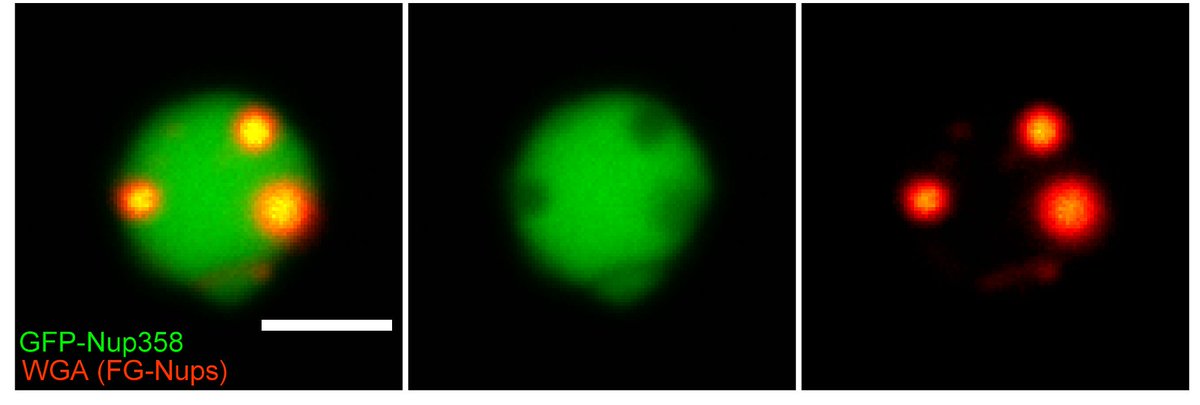
…and this is how they look like in electron microscopy. We performed correlative microscopy on an entire egg chamber and saw that they are gigantic spheres excluding ribosomes, sometimes already containing membranes packed with NPCs.
9/11
9/11
In the oocyte, we also find various possible intermediates with differing amounts of membrane content all the way up to fully stacked AL.
10/11
10/11

Finally, we looked how their interaction, condensation and resulting AL assembly are regulated and found that (similar to NPC assembly at nuclei), Ran and nuclear transport receptors such as embargoed (= Crm1 in flies) are crucial for proper assembly.
The end.
The end.

• • •
Missing some Tweet in this thread? You can try to
force a refresh