#OncoAlert Randomized cohort from CheckMate 032 now available @JTOonline. Included 243 patients with relapsed #SCLC (1-2 prior lines), no PDL1 selection. Randomized 3:2 to nivo 3mg/kg q2w or nivo 1mg/kg with ipi 3mg/kg q3w x 4 (then nivo maint). #LCSM
jto.org/article/S1556-…
jto.org/article/S1556-…
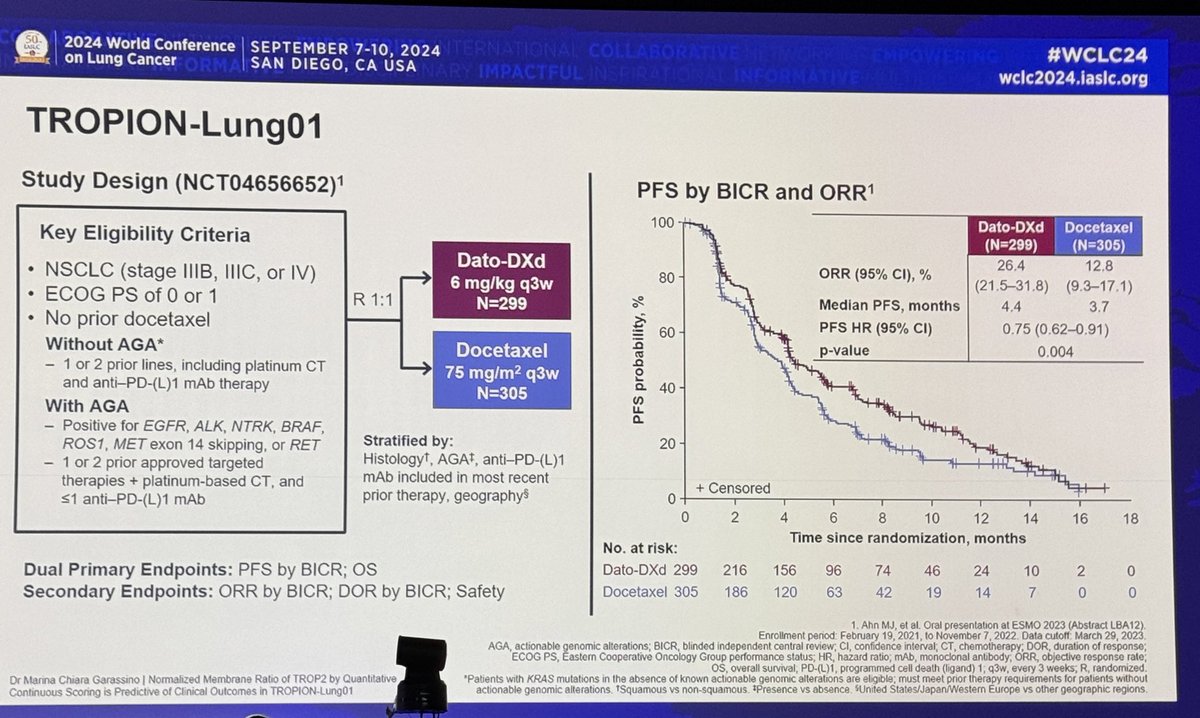
@JTOonline #OncoAlert Primary endpoint ORR (side bar: what is the best endpoint?). Early results from @ASCO 2017 showed ORR 12% nivo and 21% with nivo/ipi with improved 3 month PFS rate but no diff in 3 month OS, presented then as bar graphs instead of KM curves (?!) 

@JTOonline @ASCO #OncoAlert Now, with a median f/u of 11.9m, we see similar trends. ORR 11.6% with nivo (DOR 15.8m) and 21.9% with nivo/ipi (DOR 10m). mPFS similar (1.4 and 1.5m) but landmark PFS slightly better with nivo/ipi (3m: 32% vs 22%; 6m: 16% vs 22%, 1y: 10% vs 12%). Meaningful? #LCSM 



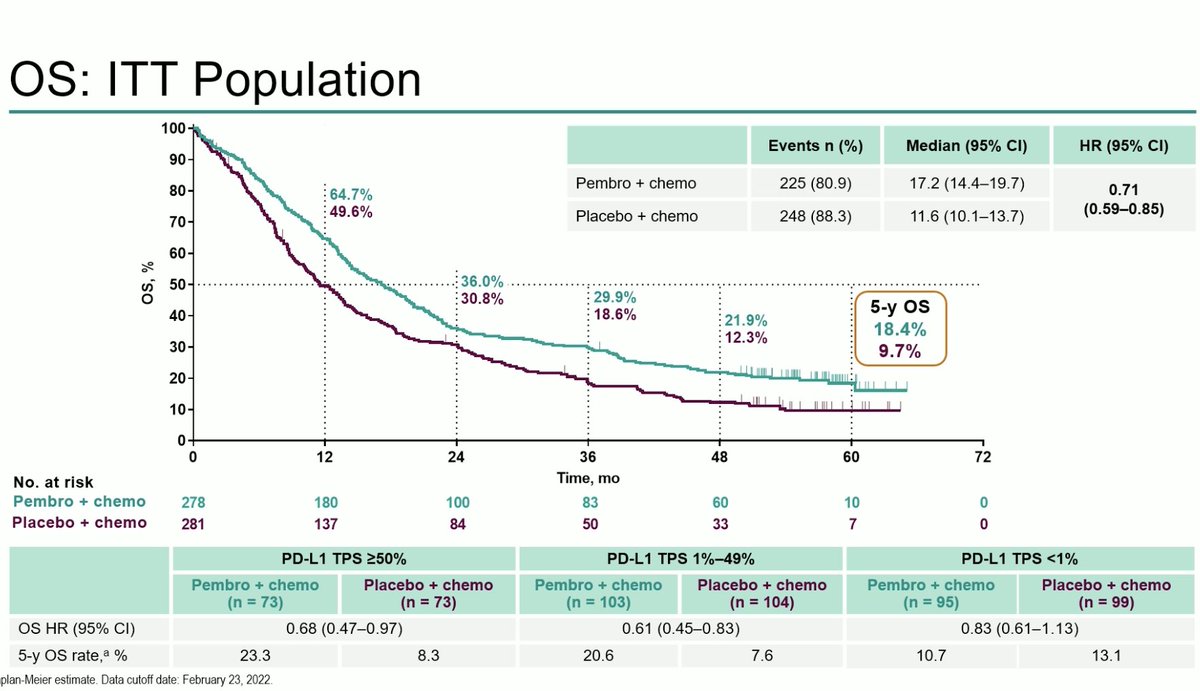
@JTOonline @ASCO #OncoAlert No difference in overall survival. mOS 5.7m with nivo and 4.7m with nivo/ipi. 12m and 24m OS rates 30.5% and 17.9% with nivo compared to 30.2% and 16.9% with nivo/ipi. No breakdown by PD-L1 status as only 24 patients had PD-L1 > 1%. And no difference by #TMB #LCSM 



@JTOonline @ASCO #OncoAlert Clear difference in safety. Grade 3-4 adverse events in 12.9% with nivo and 37.5% with nivo/ipi. Any event leading to discontinuation: 2.7% with nivo and 13.5% with nivo/ipi. Note: further therapy received in 32% nivo arm, 17% nivo/ipi. Could this be due to toxicity? 

@JTOonline @ASCO #OncoAlert Ultimately, 2y OS rate is still meaningful, just not different between the arms. Is this due to higher discontinuation with ipi? Would it be better at the low doses used in CM227? Or is there no benefit to CTLA4 in this salvage population? Clear need for biomarker.
• • •
Missing some Tweet in this thread? You can try to
force a refresh