
Thoracic Oncologist, Chief of Heme/Onc @Georgetown @MedStarGUH; Head of Developmental Therapeutics @LombardiCancer; Co-Chair #DCLung26 & #TexasLung26; #HereWeGo
How to get URL link on X (Twitter) App





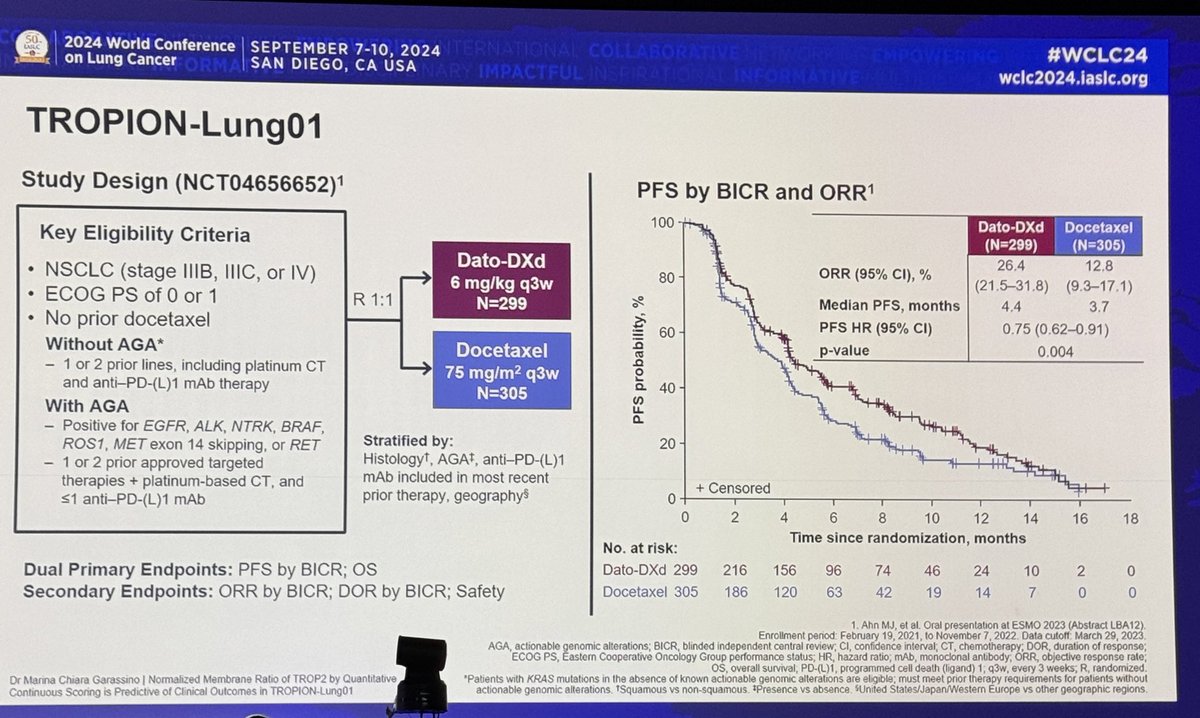 #WCLC24 TROP2 IHC has been a poor predictive marker. Normalized Membrane Ratio (NMR) factors in receptor internalization. Ratio is membrane expression over membrane plus cytoplasmic expression (using optical density from digitized slide) and lower would be more favorable.
#WCLC24 TROP2 IHC has been a poor predictive marker. Normalized Membrane Ratio (NMR) factors in receptor internalization. Ratio is membrane expression over membrane plus cytoplasmic expression (using optical density from digitized slide) and lower would be more favorable. 



 Includes resectable stage II/III (AJCC v8), EGFR/ALK wild type NSCLC. Pts receive 3 cycles of neoadjuvant chemotherapy + toripalimab vs placebo then surgery then 1 more cycle of adjuvant chemo (+tori/pbo) then 13 doses of maintenance toripalimab vs placebo. #ASCOPlenarySeries
Includes resectable stage II/III (AJCC v8), EGFR/ALK wild type NSCLC. Pts receive 3 cycles of neoadjuvant chemotherapy + toripalimab vs placebo then surgery then 1 more cycle of adjuvant chemo (+tori/pbo) then 13 doses of maintenance toripalimab vs placebo. #ASCOPlenarySeries 

 Included stage IIA-IIIB (AJCC v8) with no EGFR/ALK & excluded pts who would require pneumonectomy. Large study with n=801 (CM816 was n=358). Pts received 4 cycles (not 3) of platinum-based chemo with durvalumab (anti-PDL1) or placebo, then surgery, then durvalumab / placebo x 1y.
Included stage IIA-IIIB (AJCC v8) with no EGFR/ALK & excluded pts who would require pneumonectomy. Large study with n=801 (CM816 was n=358). Pts received 4 cycles (not 3) of platinum-based chemo with durvalumab (anti-PDL1) or placebo, then surgery, then durvalumab / placebo x 1y. 

 We're looking for synergy with the two agents - more than an additive effect. Reason to believe there will be based on preclinical data showing the effect on T-cell infiltration from #KRAS inhibition. Similar to what has been shown with MEK inhibition. #ESMOImmuno22
We're looking for synergy with the two agents - more than an additive effect. Reason to believe there will be based on preclinical data showing the effect on T-cell infiltration from #KRAS inhibition. Similar to what has been shown with MEK inhibition. #ESMOImmuno22 


 In this study of highly motivated pts at esteemed sites, evaluable paired biopsy at PD only available in 46/115 pts (40%). Interestingly, 75 pts (65%) had paired biopsy but 27 failed NGS (23%). Speaks somewhat to the real world feasibility of a repeat biopsy approach. #ESMO22
In this study of highly motivated pts at esteemed sites, evaluable paired biopsy at PD only available in 46/115 pts (40%). Interestingly, 75 pts (65%) had paired biopsy but 27 failed NGS (23%). Speaks somewhat to the real world feasibility of a repeat biopsy approach. #ESMO22 

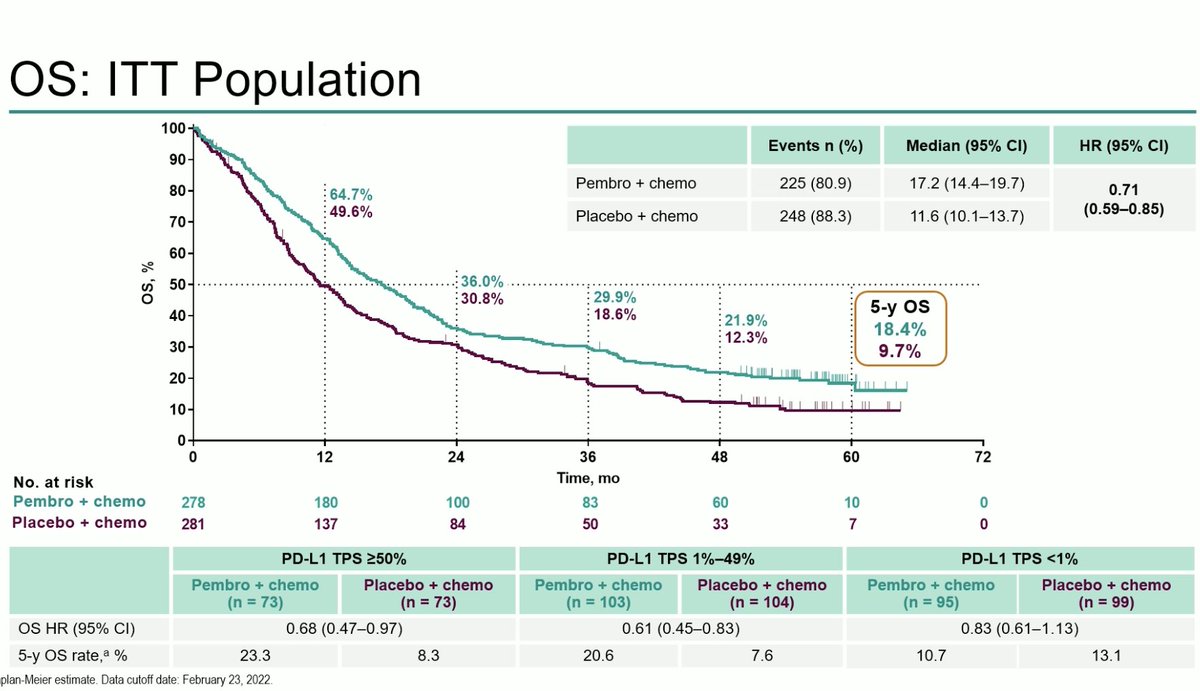
 With longer follow up, OS favors pembrolizumab arm with mOS 17.2 vs 11.6m (OS HR 0.71) in squamous NSCLC. 5y OS rate 18.4% vs 9.7%. PFS benefit (HR 0.62) and higher RR across PDL1 strata. #ESMO22
With longer follow up, OS favors pembrolizumab arm with mOS 17.2 vs 11.6m (OS HR 0.71) in squamous NSCLC. 5y OS rate 18.4% vs 9.7%. PFS benefit (HR 0.62) and higher RR across PDL1 strata. #ESMO22 



 KEYNOTE 189 is our SOC and has shown a consistent benefit including improving OS even with a crossover rate of 57%. #ESMO22
KEYNOTE 189 is our SOC and has shown a consistent benefit including improving OS even with a crossover rate of 57%. #ESMO22 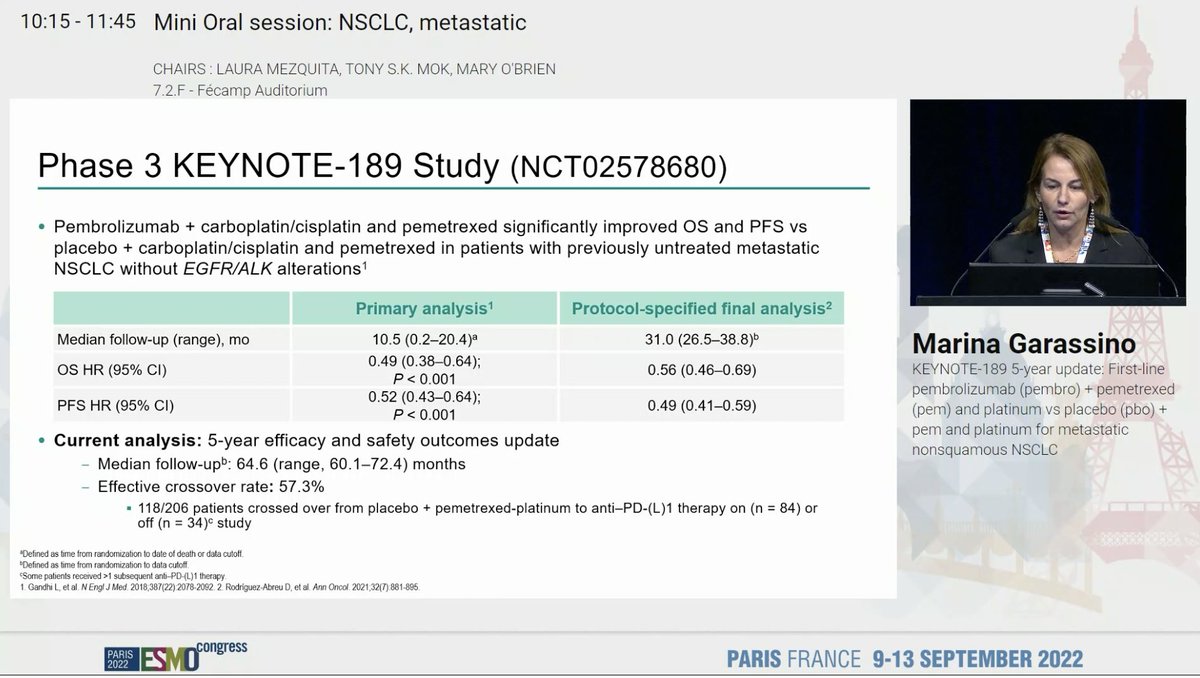

 Study design here shows randomization to first-line sintilimab + anlotinib or chemo (with sintilimab at progression). Some concerns about randomization to chemotherapy without immunotherapy in a modern day study. #ESMO22
Study design here shows randomization to first-line sintilimab + anlotinib or chemo (with sintilimab at progression). Some concerns about randomization to chemotherapy without immunotherapy in a modern day study. #ESMO22 


 Trastuzumab deruxtecan (T-DXd) is an antibody drug conjugate (ADC) targeting #HER2 and in DESTINY-Lung01, RR was encouraging at 55% but some concern for toxicity (adjudicated drug-related ILD in 26% of cases). #ESMO22
Trastuzumab deruxtecan (T-DXd) is an antibody drug conjugate (ADC) targeting #HER2 and in DESTINY-Lung01, RR was encouraging at 55% but some concern for toxicity (adjudicated drug-related ILD in 26% of cases). #ESMO22 

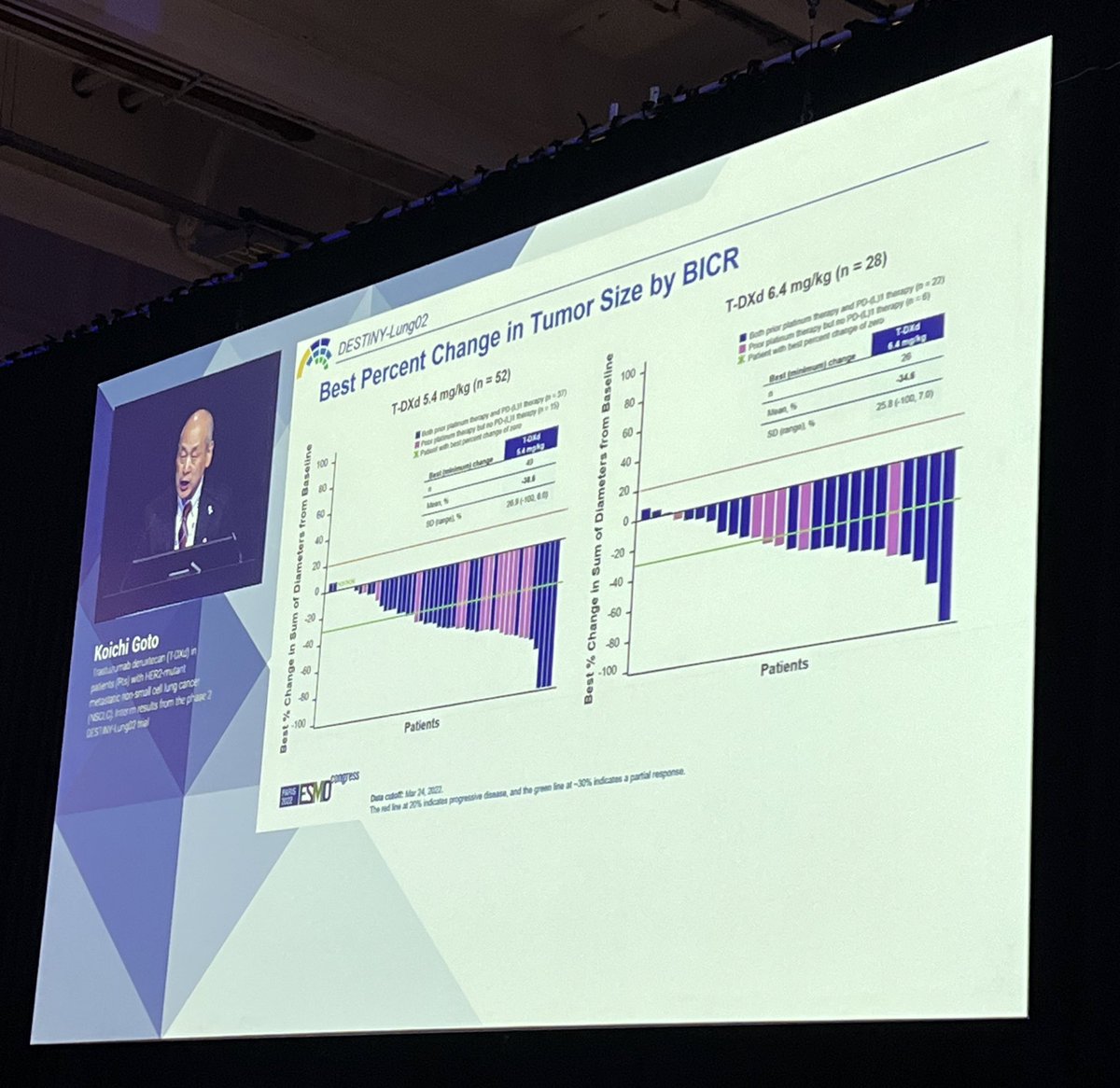
 DESTINY-Lung02 compared two doses of trastuzumab deruxtecan (T-DXd) in #HER2 previously treated NSCLC (5.4 mg/kg vs 6.4 mg/kg): RR 58% and DoR 8.7m at the lower dose compared to 43% at 6.4 mg/kg. #ESMO22
DESTINY-Lung02 compared two doses of trastuzumab deruxtecan (T-DXd) in #HER2 previously treated NSCLC (5.4 mg/kg vs 6.4 mg/kg): RR 58% and DoR 8.7m at the lower dose compared to 43% at 6.4 mg/kg. #ESMO22 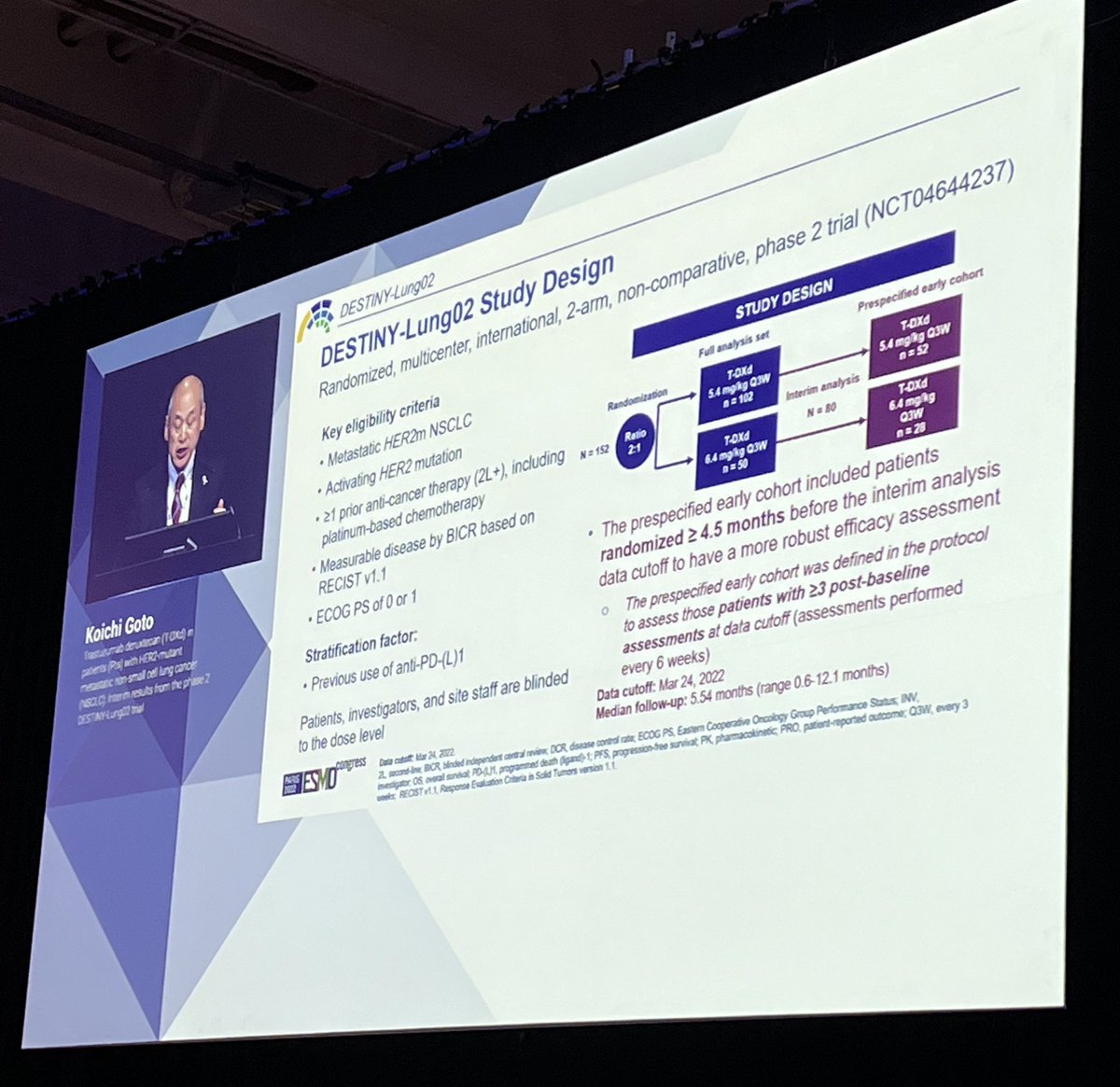



 The difference here is radiation and in T3/T4 NSCLC, local control is so critical. Largely unexplored to date. The INCREASE study explores quad-modality therapy and the results were quite impressive. High pCR rates and seen across PDL1 strata, not just in PDL1 high. #ESMO22
The difference here is radiation and in T3/T4 NSCLC, local control is so critical. Largely unexplored to date. The INCREASE study explores quad-modality therapy and the results were quite impressive. High pCR rates and seen across PDL1 strata, not just in PDL1 high. #ESMO22 




 Focus is on high T stage and low N stage - not resectable initially but potentially resectable after therapy. Patients here received nivo/ipi on day 1 and nivo on day 22 with chemoradiation then resection 6w later (after restaging) with pCR/MPR primary endpoint. #ESMO22
Focus is on high T stage and low N stage - not resectable initially but potentially resectable after therapy. Patients here received nivo/ipi on day 1 and nivo on day 22 with chemoradiation then resection 6w later (after restaging) with pCR/MPR primary endpoint. #ESMO22 


 @DrSanjayPopat @myESMO #ESMO22 @DrSanjayPopat points out that the DFS HR is on par with the original analysis but the shape of the curves is different, especially for stage II/III
@DrSanjayPopat @myESMO #ESMO22 @DrSanjayPopat points out that the DFS HR is on par with the original analysis but the shape of the curves is different, especially for stage II/III 



 The DFS HR of 0.23 in resected stage II/III remains very impressive. But once osimertinib is stopped after 3y, the curves do seem to be coming closer together. DFS at 2y with osi is 90% and at 3y is 84% but drops to 70% at 4y. Are we preventing recurrence or delaying it? #ESMO22
The DFS HR of 0.23 in resected stage II/III remains very impressive. But once osimertinib is stopped after 3y, the curves do seem to be coming closer together. DFS at 2y with osi is 90% and at 3y is 84% but drops to 70% at 4y. Are we preventing recurrence or delaying it? #ESMO22 

 #WCLC20 Amivantamab is an EGFR-MET bispecific antibody given intravenously weekly x 1 month then q2w. This is a single arm phase II for patients with #EGFRex20 mutant NSCLC after prior chemotherapy. #LCSM
#WCLC20 Amivantamab is an EGFR-MET bispecific antibody given intravenously weekly x 1 month then q2w. This is a single arm phase II for patients with #EGFRex20 mutant NSCLC after prior chemotherapy. #LCSM 



 #WCLC20 This study had two cohorts. Here, we focus on Cohort 1 (HER2-overexpressing = IHC3+ or IHC2+). Primary response rate was ORR. #LCSM
#WCLC20 This study had two cohorts. Here, we focus on Cohort 1 (HER2-overexpressing = IHC3+ or IHC2+). Primary response rate was ORR. #LCSM 



 #WCLC20 Cohort being discussed here is #EGFR exon 20 insertion NSCLC after prior platinum therapy and the EXCLAIM extension cohort. Many patients also had prior TKI therapy and prior immunotherapy. @IASLC
#WCLC20 Cohort being discussed here is #EGFR exon 20 insertion NSCLC after prior platinum therapy and the EXCLAIM extension cohort. Many patients also had prior TKI therapy and prior immunotherapy. @IASLC 





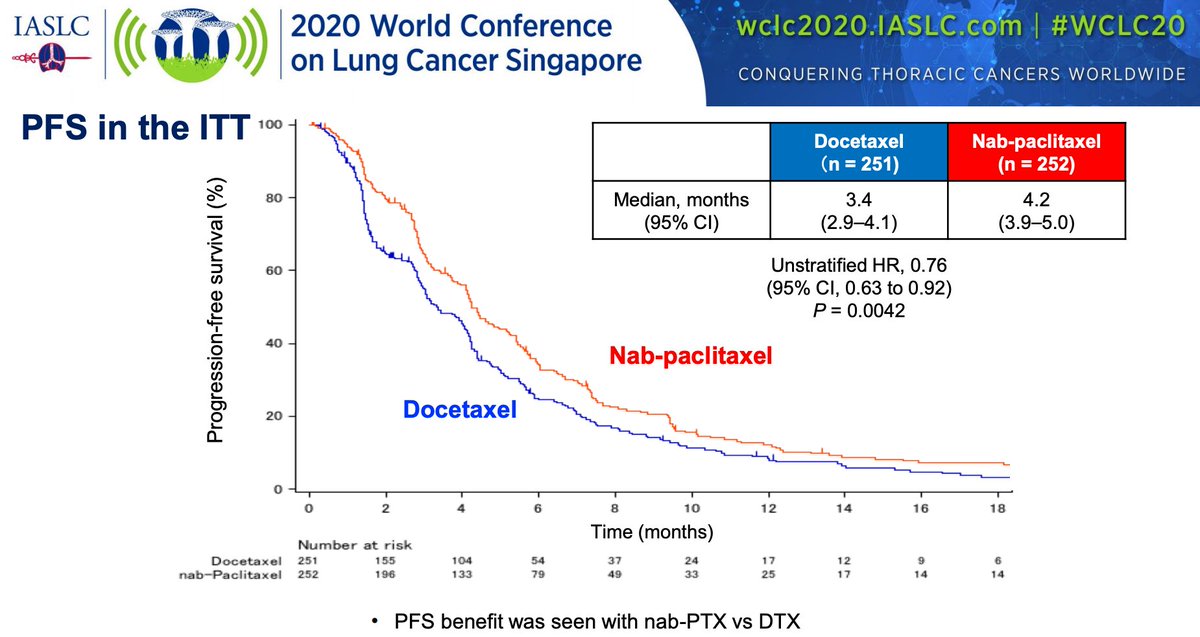
 #WCLC20 Positive study! Shows non-inferiority in OS with nab-paclitaxel over docetaxel (median 16.2m vs 13.6m). PFS favors nab-pac (HR 0.76). #LCSM
#WCLC20 Positive study! Shows non-inferiority in OS with nab-paclitaxel over docetaxel (median 16.2m vs 13.6m). PFS favors nab-pac (HR 0.76). #LCSM 

 #WCLC20 Presentation here is on the combined dose escalation and expansion at the 5.6 mg/kg dose with #EGFR mutant NSCLC. Includes 56 evaluable patients. Median f/u 5 months. #LCSM @IASLC
#WCLC20 Presentation here is on the combined dose escalation and expansion at the 5.6 mg/kg dose with #EGFR mutant NSCLC. Includes 56 evaluable patients. Median f/u 5 months. #LCSM @IASLC 



 #WCLC20 This ADC includes an anti-TROP2 IgG1 and a topo-1 inhibitor payload. Phase 1 design shown here. Mostly non-squamous, included some #EGFR+, heavily pretreated including many with CNS metastases. Less intensity at higher 8mg dose, as one would expect. #LCSM
#WCLC20 This ADC includes an anti-TROP2 IgG1 and a topo-1 inhibitor payload. Phase 1 design shown here. Mostly non-squamous, included some #EGFR+, heavily pretreated including many with CNS metastases. Less intensity at higher 8mg dose, as one would expect. #LCSM 


