#OncoAlert #AACR20 Results from phase II study of camrelizumab (SHR-1210, anti-PD-1) plus apatinib in #SCLC presented by Dr. Jie Wang. Camrelizumab is approved in China for Hodgkin lymphoma and has shown promise in HCC. Here, results from single arm phase II. #LCSM 

#OncoAlert #AACR20 Stage I included three different schedules of apatinib delivery (camrelizumab is given IV q2w). The apatinib 375mg qday schedule was chosen for stage 2. Included chemo-sensitive and resistant #SCLC, using a 90-day platinum-free interval cutoff. #LCSM 

#OncoAlert #AACR20 Patient characteristics reveal a slightly higher proportion of never-smokers (10/47, 21%). #SCLC strongly linked to smoking but can be seen in never smokers (4.5% in IMpower 133). Spontaneous transformation from #EGFR? Different outcomes in this group? #LCSM 

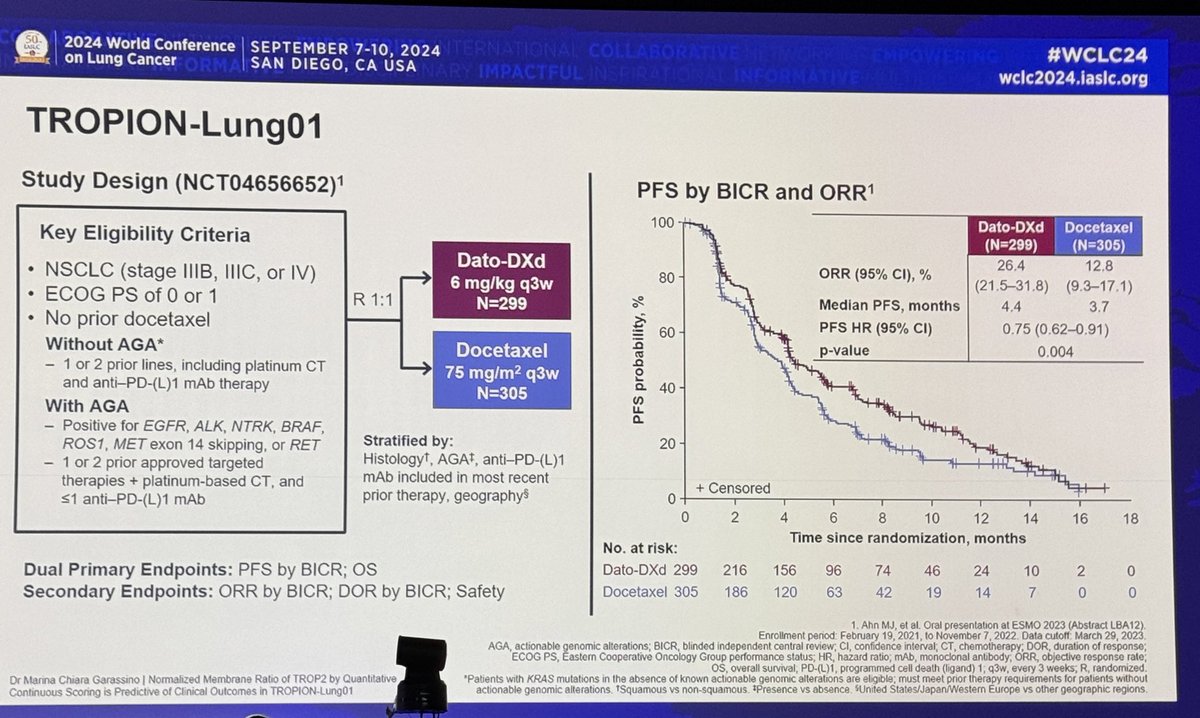
#OncoAlert #AACR20 Camrelizumab plus apatinib qday had a RR of 34% including a 32.3% RR in chemo-resistant #SCLC. Encouraging activity. Median duration of response 6.2 months with many ongoing responses. Recall 3L nivo RR 11.9%, 3L pembro RR 19.3%. Is this notably better? #LCSM 



#OncoAlert #AACR20 RR encouraging but hard to interpret single arm phase II. What is the contribution of apatinib? VEGFR2 + PD1 is an exciting combination but would we have seen the same results (but less tox) with camrelizumab alone? Need a comparator arm. #LCSM
#OncoAlert #AACR20 Adverse events included #VEGF-related tox. How does this apply in IMp133/CASPIAN world where IO is delivered with 1L therapy? I would welcome more novel agents for #SCLC, but hoping to see biomarker work from this trial and a follow up randomized effort. #LCSM 

• • •
Missing some Tweet in this thread? You can try to
force a refresh