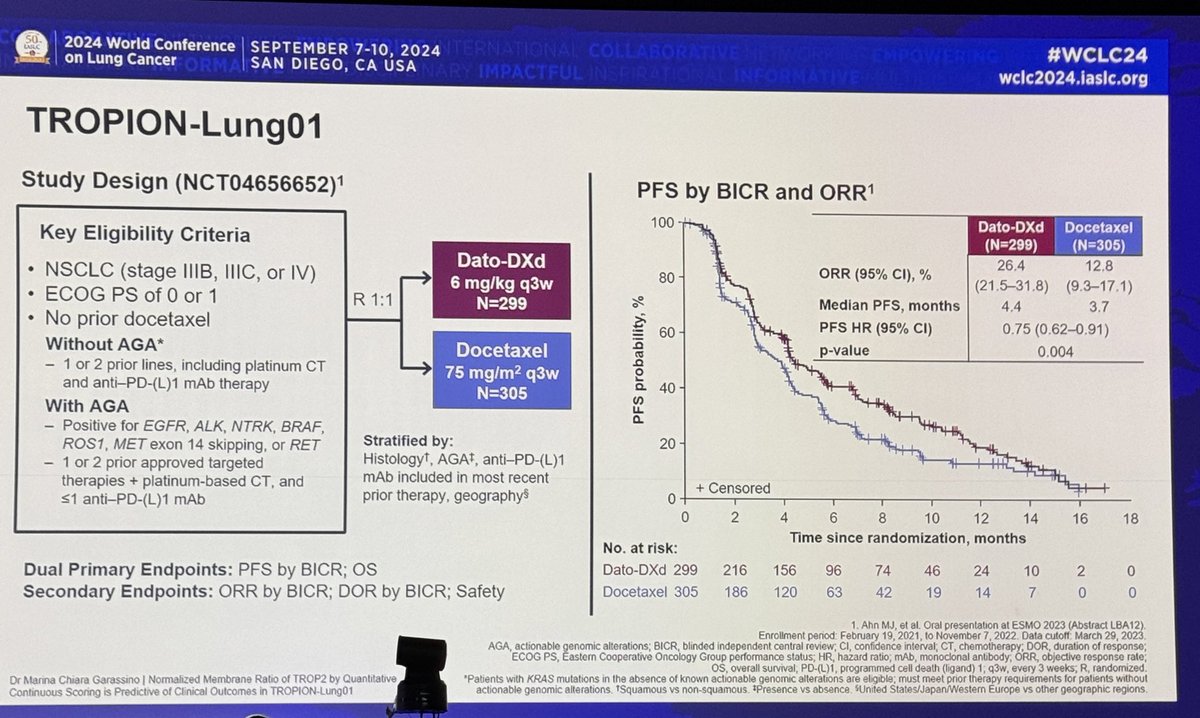
#ASCO20 Important data for #HER2 #NSCLC from the DESTINY-Lung01 study of trastuzumab deruxtecan (T-DXd, DS-8201) in previously treated, HER2 mutant NSCLC. T-DXd is an antibody drug conjugate (ADC) with a topo-I inhibitor payload linked to a trastuzumab-like IgG1 mAb. #OncoAlert 





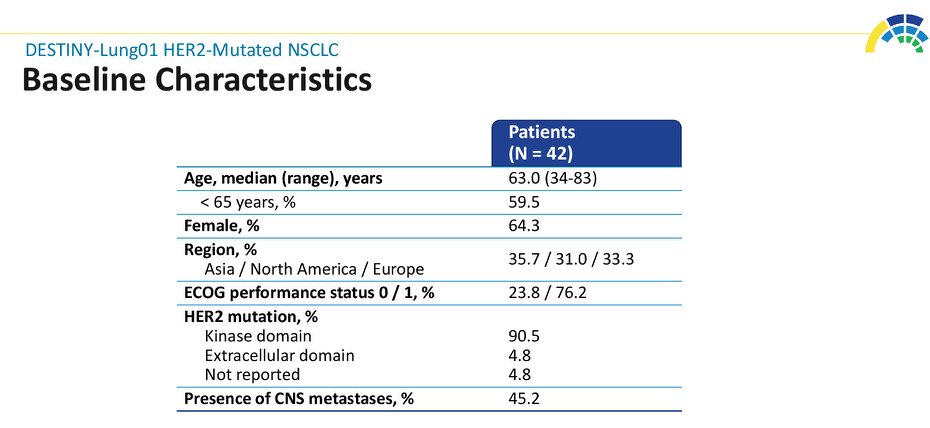
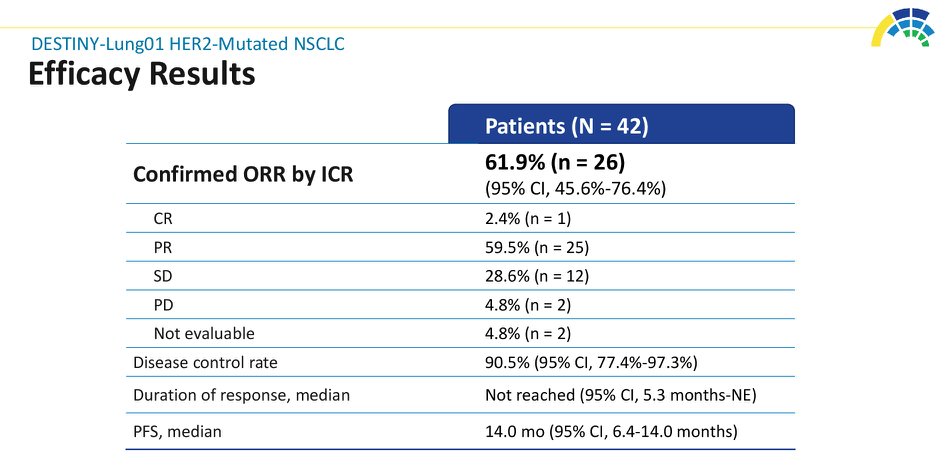
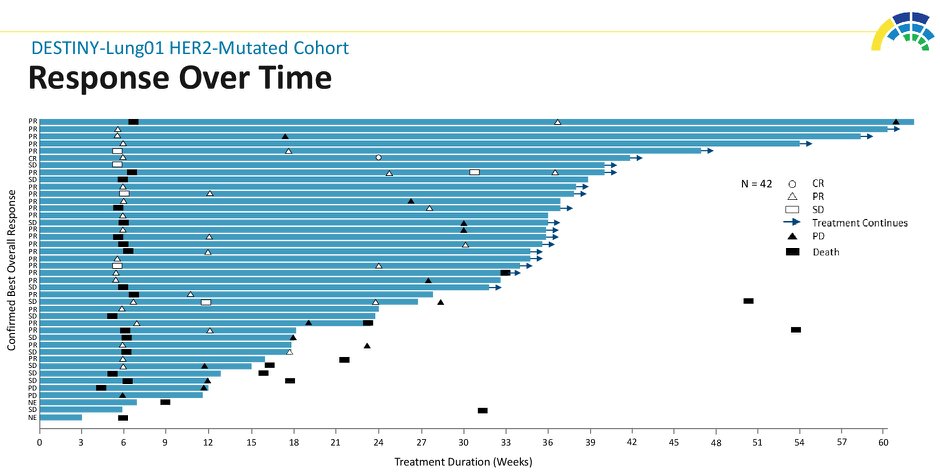
#ASCO20 Included 42 pts with #HER2 mut #NSCLC (45% with CNS metastases) treated with T-DXd 6.4mg/kg q3w. Impressive outcomes: ORR 62%, DCR 90%, mPFS 14 months! And 45% of patients still on treatment. #OncoAlert #LCSM 




#ASCO20 T-DXd led to nausea in most patients (largely G1-2). Alopecia in about half; anemia and neutropenia (some G3+) also observed. Treatment emergent AEs leading to discontinuation in 24% of patients. Grade 2 interstitial lung disease seen in 12% (median onset 86d). #OncoAlert 





• • •
Missing some Tweet in this thread? You can try to
force a refresh