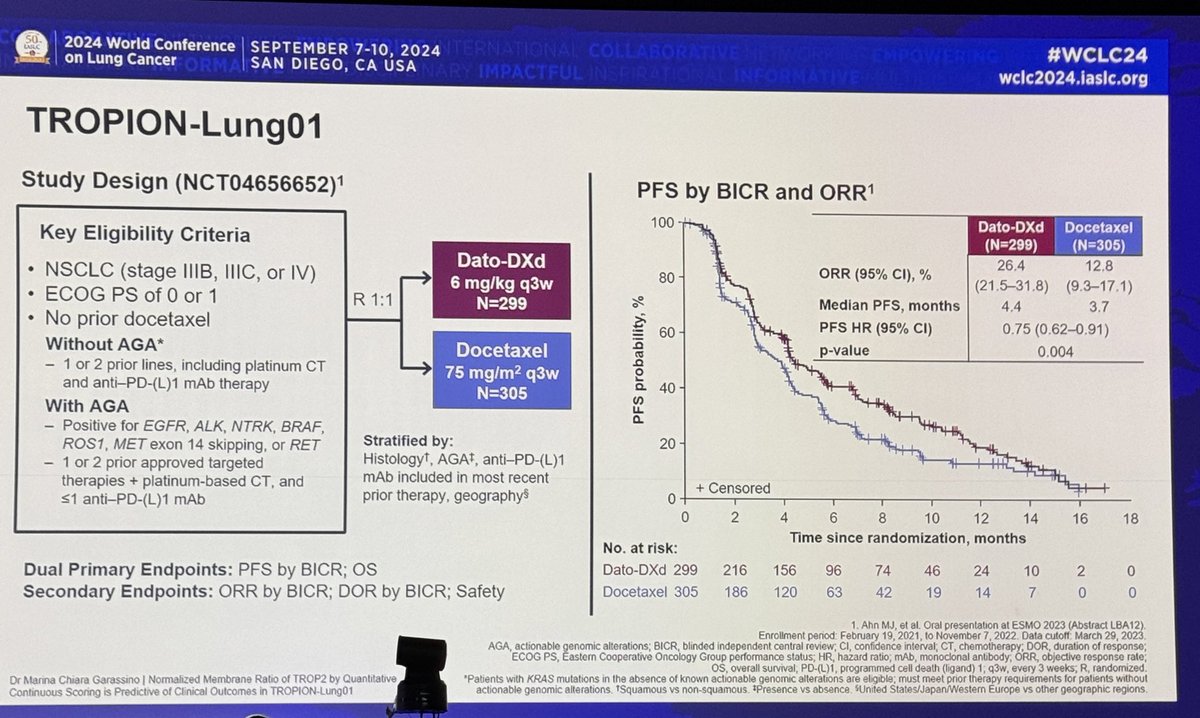
#ASCO20 Very promising results from PrECOG 0505 trial presented by @FordePatrick: Single arm phase II of durvalumab with cis/pem as 1L treatment for unresectable pleural mesothelioma (#MPM) #OncoAlert 

#ASCO20 PrE0505 enrolled 55 patients (75% epithelioid) in 1y and treated with 6 cycles of standard cis/pem with durva 1120mg q3w followed by durva maintenance therapy (until PD). #OncoAlert 



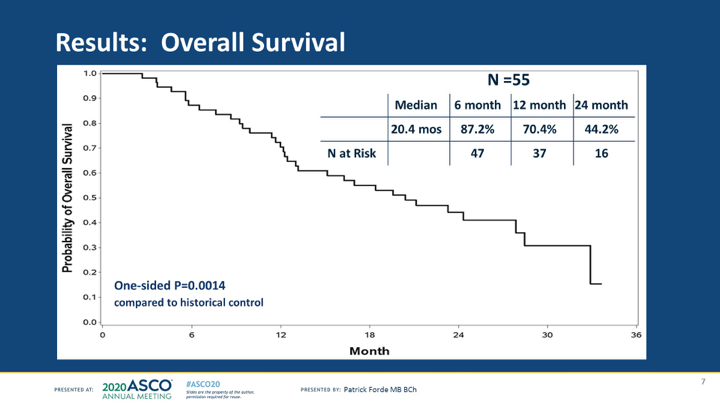
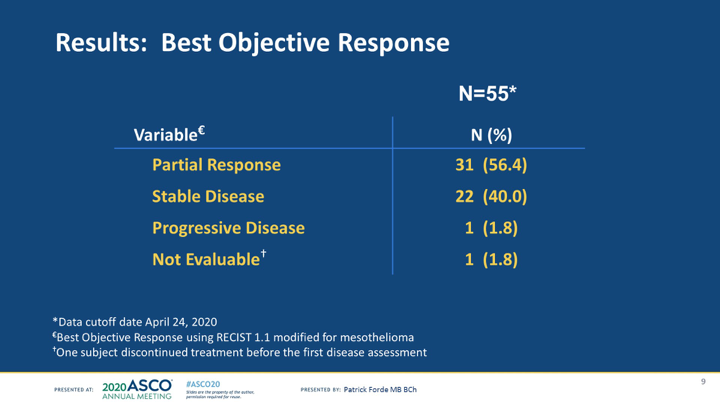
#ASCO20 Impressive OS numbers here with addition of durvalumab to cis/pem in #MPM with median of 20.4m and a 1y OS rate of 70.4%. Median PFS was 6.7m and RR was 56% (in the Australian DREAM trial RR was 48%). #OncoAlert 





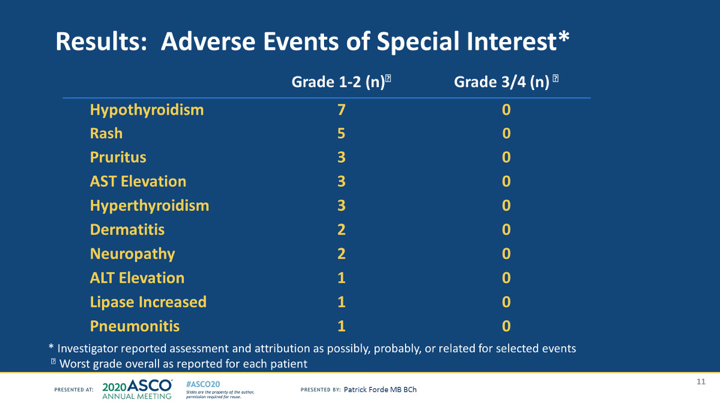
#ASCO20 Toxicity with durva + cis/pem primarily chemo-related toxicity, myelosuppression, low rates of immune mediated AEs. No clear associations with #TMB or #PDL1 expression. Phase III trial (Pr0506/DREAM3R) planned for later this year. #OncoAlert 




#ASCO20 Great results! But just to provide balance: OS with durva/cis/pem of 20.4m better than cis/pem in MAPS trial of 16m. But cis/pem in the Vogelzang study had OS 12m. Expect some rise in OS in a more modern era (perils of cross trial comparison). Need the ph III! #OncoAlert 

• • •
Missing some Tweet in this thread? You can try to
force a refresh