#ASCO20 Highly anticipated plenary presentation of #ADAURA by @DrRoyHerbstYale: 3y of adjuvant osimertinib after resection of #EGFR+ NSCLC #OncoAlert #LCSM 

#ASCO20 In the advanced setting, #EGFR TKI therapy is our clear standard. Relatively rapid implementation and the current SOC for stage IV NSCLC is osimertinib. #LCSM #OncoAlert 
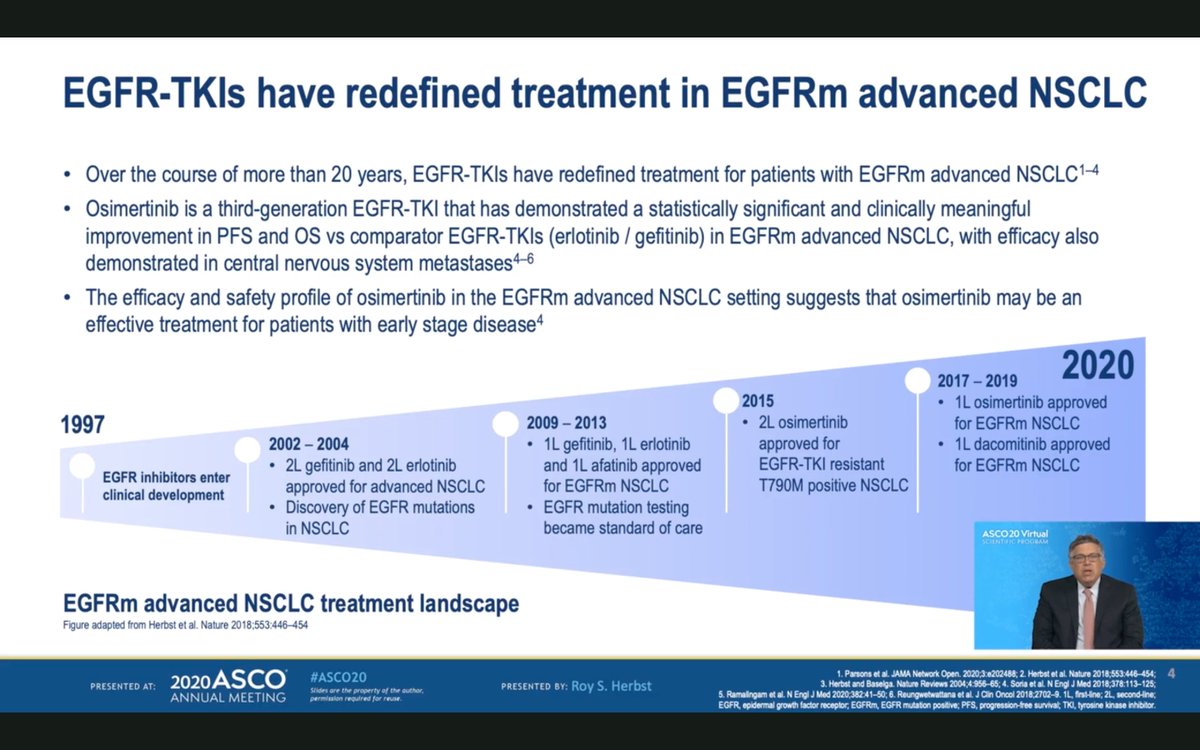
#ASCO20 The phase III #ADAURA trial included patients with resected stage IB, II or IIIA NSCLC (with or without adjuvant chemotherapy) with an #EGFR deletion 19 or L858R mutation. CNS imaging and post-op CT required before entry. #OncoAlert #LCSM 

#ASCO20 Study design very straightforward with stratifiation by stage, mutation and race. Randomized to osimertinib 80mg qd vs placebo for 3 years (or progression). Stopped early by IDMC recommendation. Note: min f/u only 1 year, most had 2y of f/u. #OncoAlert #LCSM 

#ASCO20 Baseline characteristics show about 55% of patients received adjuvant chemotherapy. 65% Asian, 70% non-smoker, 55% #EGFR del19. #OncoAlert #LCSM 

#ASCO20 DFS for patients with stage II/IIIA (primary endpoint) HR 0.17! Only 33% mature but impressive landmark DFS rates: 90% with osimertinib at 2y vs 44% with placebo. Early separation of the curves, consistent with prior reports. Surgery often not curative in this setting. 

#ASCO20 Forest plot for DFS shows impressive HR in every major subgroup, though IB has a large CI. HR for those who received adjuvant chemo was 0.18 and for those who did not 0.23 #OncoAlert #LCSM 

#ASCO20 #ADAURA DFS rate by stage showing improvement across the board, moreso in stage II (HR 0.17) and stage III (HR 0.12) than stage IB (HR 0.50). #OncoAlert #LCSM 

#ASCO20 DFS KM curves by stage all show early separation, impressive HR. A reminder of how few patients with stage II and especially stage IIIA EGFR+ NSCLC we actually cure with surgery +/- chemotherapy. #OncoAlert #LCSM 

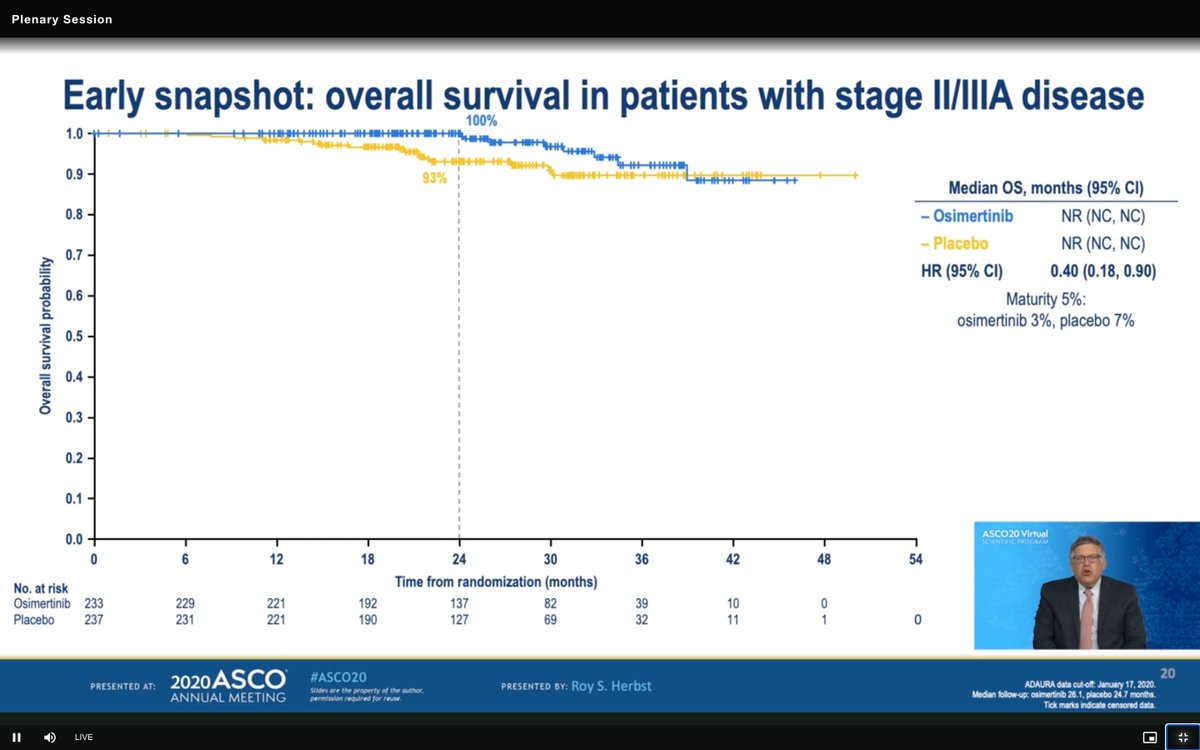
#ASCO20 Very early and immature OS curves for #ADAURA. This will be the key. Note that this HR of 0.40 (95% CI 0.18, 0.90) is not statistically valid at this point of maturity. We will be watching this closely in the years to come (5% maturity). #OncoAlert #LCSM 
#ASCO20 Safety summary here and critical for an adjuvant setting. Overall appears quite safe but our bar is higher in patients who may not have active disease and without a clear OS benefit yet. And this is not 4 cycles - this is 3 years. QOL is very important. #OncoAlert #LCSM 

#ASCO20 Tornado plot illustrates osimertinib safety and while rate of G3+ AEs are quite low, 46% with all grade diarrhea is notable (again, 3y of therapy). #OncoAlert #LCSM 

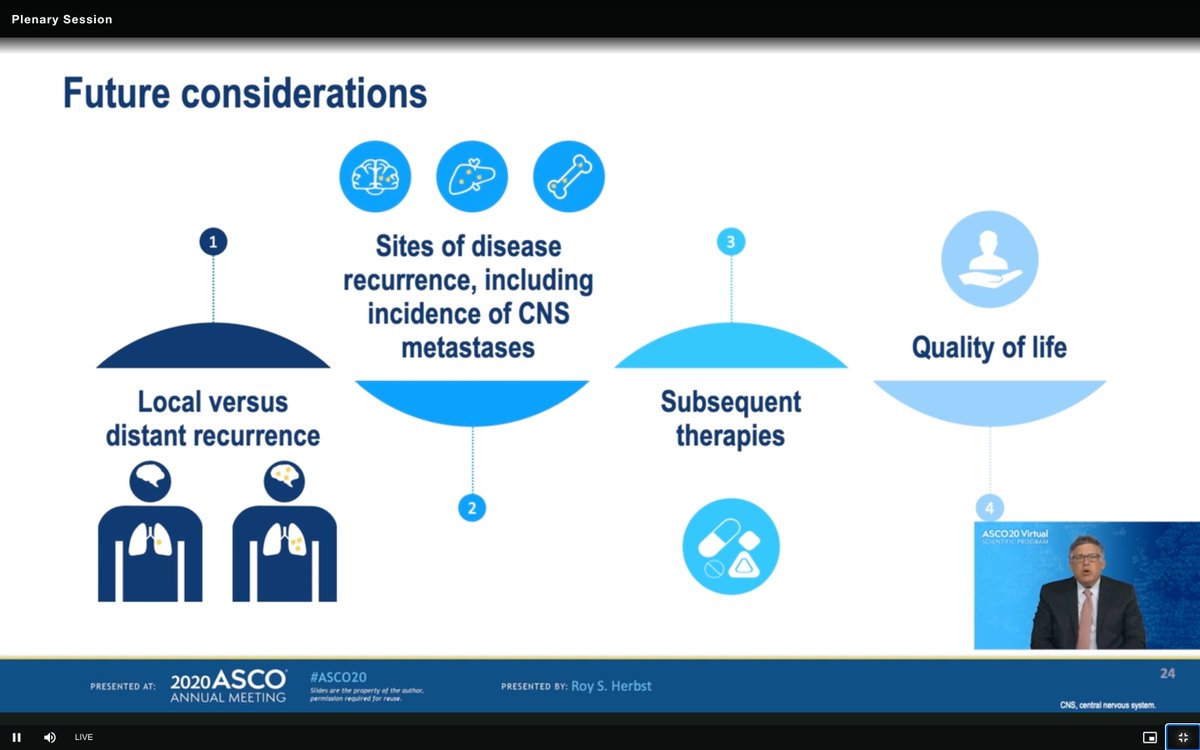
#ASCO20 Breakdown of future considerations for #ADAURA by @DrRoyHerbstYale - glad to see QOL and capture of subsequent therapies (how many in placebo receive osi at PD) and characterization of PD. Will also need to see how many crossover after unblinding... #OncoAlert #LCSM 
#ASCO20 Overall, DFS HR of 0.21 across stages. 2y DFS rate 89% with osimertinib vs 53% with placebo. Immature, yes. Perfect dataset, no. But impressive nonetheless. #OncoAlert #LCSM 

• • •
Missing some Tweet in this thread? You can try to
force a refresh