Atezolizumab + Bevacizumab in Unresectable HCC (IMbrave150 trial) by Dr. Richard Finn et al.
Expert opinion: @SeragHashem
Moderators: @AtoosaRabiee @jturnesv
nejm.org/doi/full/10.10…
#livertwitter #GIJC #hpbcsm
- Sorafenib in 2008: improved OS + longer time to radiologic progression vs placebo
nejm.org/doi/full/10.10…
Single agent PD-L1 inhibitor w/o improved OS
- Nivoumab vs sorafenib: No statistically significant improvement in OS w/nivolumab
sciencedirect.com/science/articl…
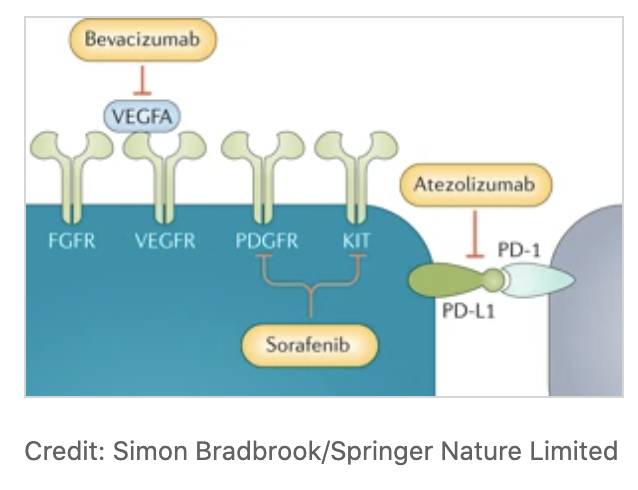
- Atezolizumab: a programmed death ligand 1 (PD-L1) inhibitor
- Bevacizumab: a monoclonal antibody targeting the VEGF
- Sorafenib: multikinase inhibitor
Picture credit: nature.com/articles/s4157…
- ECOG score 2-5
- Child Pugh Class B or C
- History of autoimmune disease
- Co-infection w/HBV or HCV
- Untreated esophageal or gastric varices [all patients enrolled had an EGD w/in 6 months of enrollment, given association b/w VEGF therapy & bleeding risk]
- Overall survival (OS)
- Progression free survival (PFS)
Secondary endpoints:
- Objective response rate
- Duration of response
- Time to deterioration of quality of life, physical functioning, and role functioning
📈Analysis: Intention to treat
- Open label, but a blinded independent review of imaging was done to reduce bias
- CTP B & C were excluded & all patients had an EGD w/in 6 months. The safety of combo tx in more decompensated patients needs further study
- Supported by F. Hoffmann–La Roche/Genentech
Atezolizumab plus bevacizumab (vs sorafenib) resulted in significantly better overall survival and progression free survival in patients with unresectable HCC without prior systemic treatment
nejm.org/doi/full/10.10…
#livertwitter #GIJC



















