#OncoAlert Online now @JTOonline is the ORIENT-11 trial of sintilimab + platinum/pemetrexed in NSCLC. Simultaneous publication with #VPS20 #WCLC20 @IASLC presentation. Randomized phase III study of chemotherapy +/- anti PD-1 antibody sintilimab. #LCSM
jto.org/article/S1556-…
jto.org/article/S1556-…
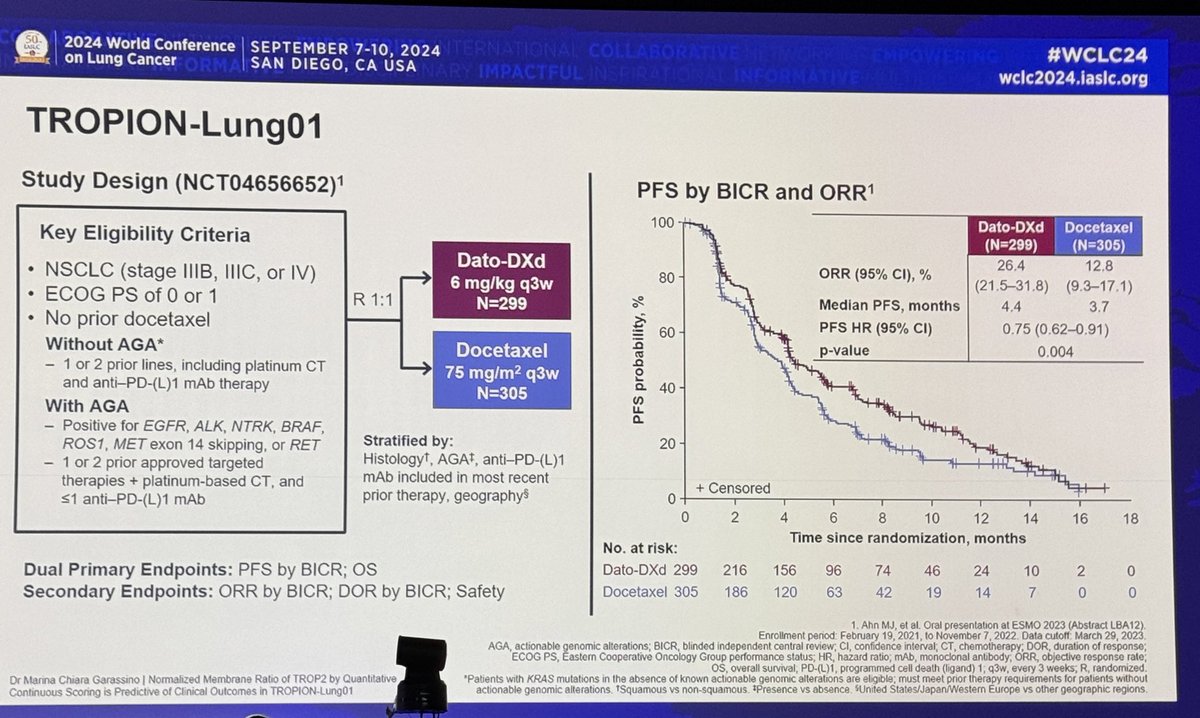
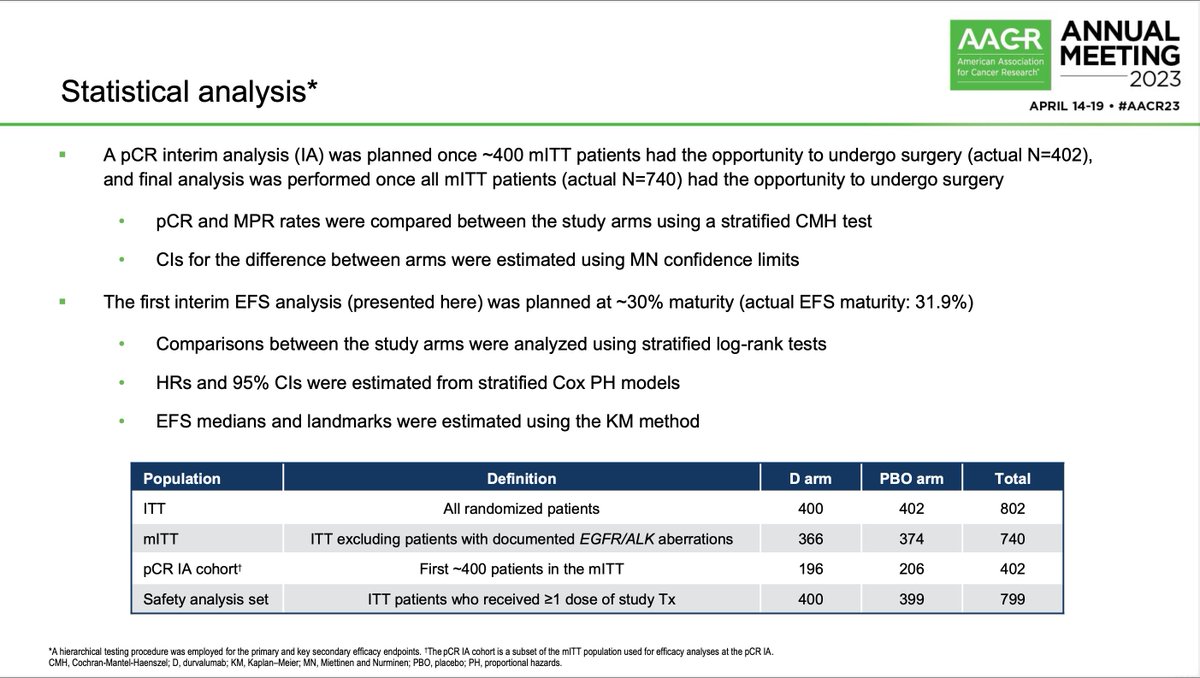
Sintilimab is a PD1 antibody and the phase Ib of sintilimab with platinum/pem had RR 68.4% and mPFS 11.4m. Phase III included 397 patients in 47 hospitals in China with treatment naive advanced EGFR/ALK wild type non-squamous NSCLC, stratified by PDL1 expression (22C3). 

Enrolled in < 1y! All patients received 4 cycles of carboplatin AUC 5 with pemetrexed 500mg/m2 q21d and randomized 2:1 to sintilimab 200mg flat dose or placebo followed by sintilimab/placebo maintenance. After median f/u of 8.9m, primary endpoint met: PFS 8.9m vs 5m (HR 0.482). 
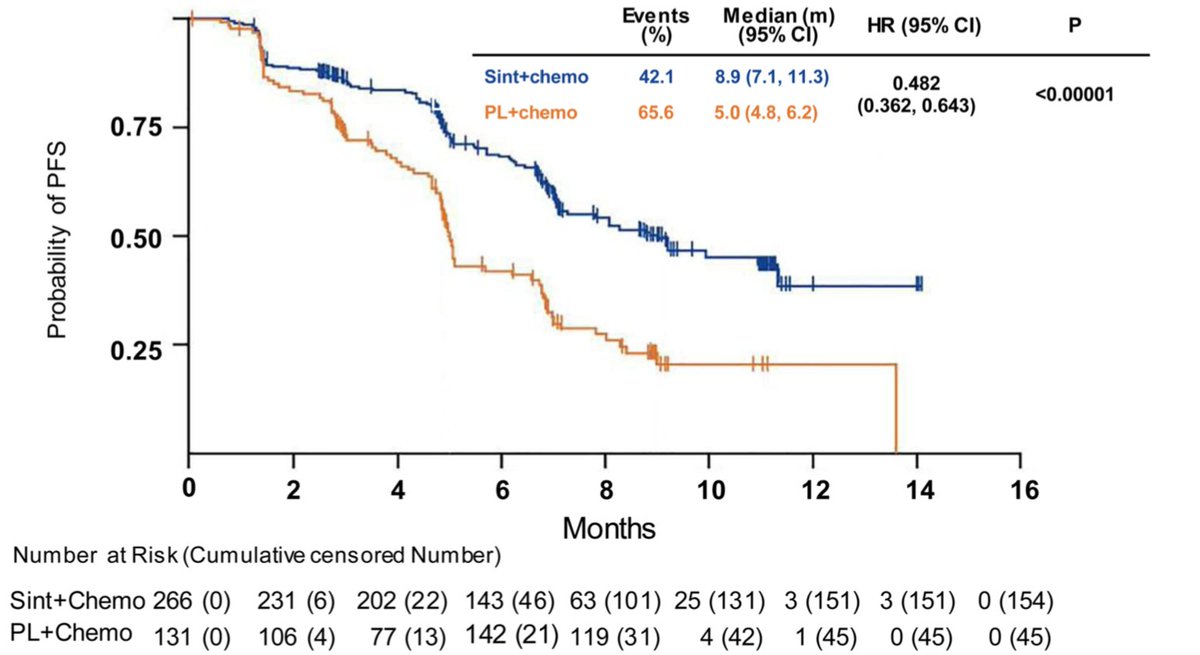
Early split and no crossing of curves, very comparable to KEYNOTE-189. Benefit across major subgroups (including non-smokers), stepwise benefit by PDL1 expression. Consistent results; good to demonstrate in East Asian population (only 1% of patients receiving pembro in KN189). 



Benefit seen across PDL1 strata. In PDL1 negative, PFS 7.3 vs 5.1m (HR 0.664). In PDL1 low, PFS 7.1 vs 4.8 (HR 0.503). In PDL1 high, PFS NR vs 5m (HR 0.310). RR higher with sintilimab(51.9% vs 29.8%) and median time to response 1.5m. 



No worrisome safety signals with comparable G3+ toxicity in both arms (primarily due to chemotherapy). Discontinuation of sintilmab due to AE seen in 6% (vs 8.4% in placebo arm). irAEs in 43.2% with sintilmab and 36.6% with placebo (blinded study), but mostly G1-2. 

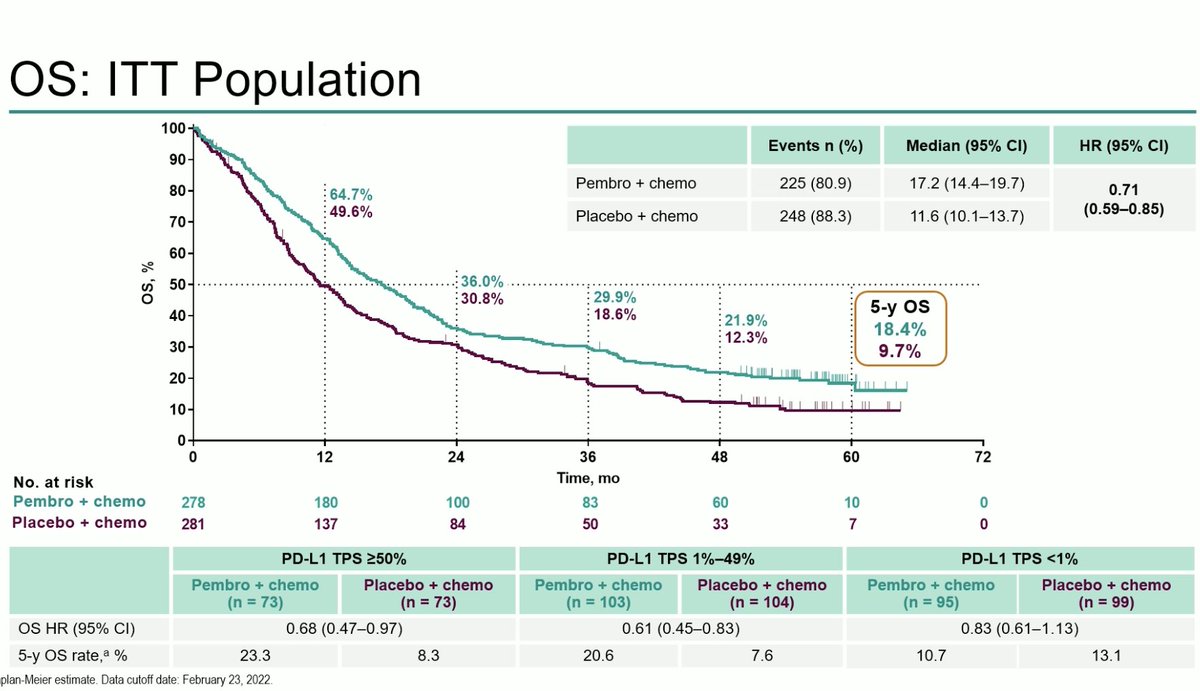
OS is immature with medians not reached but HR 0.609 trending to favoring sintilimab. Crossover permitted but only 31.3% in control arm crossed over to sintilimab (another 4.6% received an alternate immunotherapy). #LCSM #OncoAlert @IASLC @JTOonline 

• • •
Missing some Tweet in this thread? You can try to
force a refresh