Cases of primary polydipsia in patients with psychiatric illnesses have been reported for decades.
While thirst isn't always mentioned as the primary driver, it is present often enough to suspect it has a role.
pubmed.ncbi.nlm.nih.gov/14185641/
pubmed.ncbi.nlm.nih.gov/1551044/

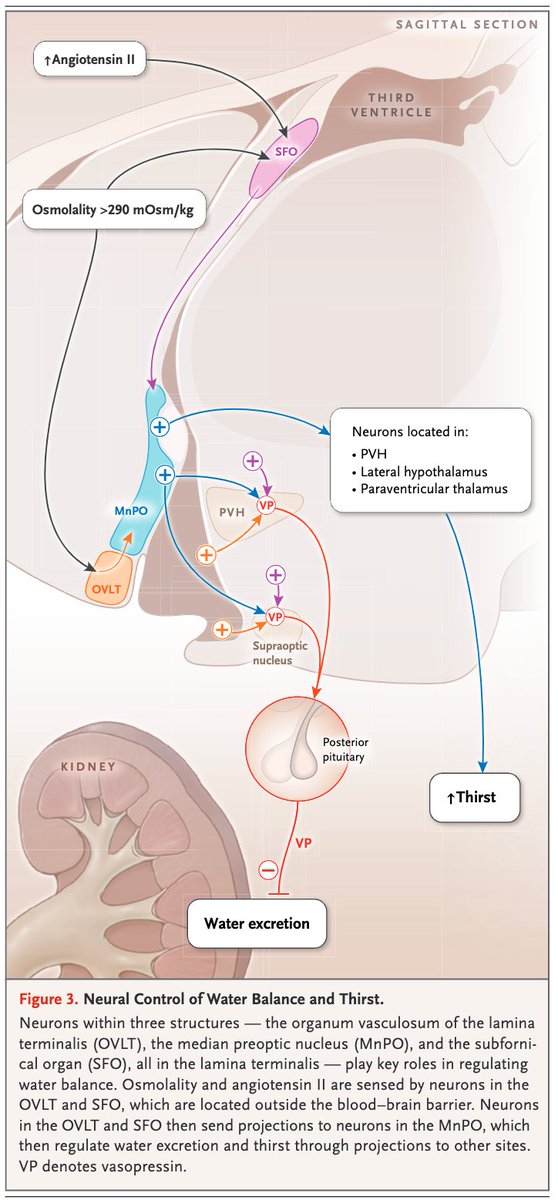
One driver of thirst is osmolarity.
As osmolarity increases, the subfornical organ (SFO) and the organum vasculosum of the lamina terminalis (OVLT) sense these changes and send projections to other brain structures.
The result: thirst
pubmed.ncbi.nlm.nih.gov/30699320/
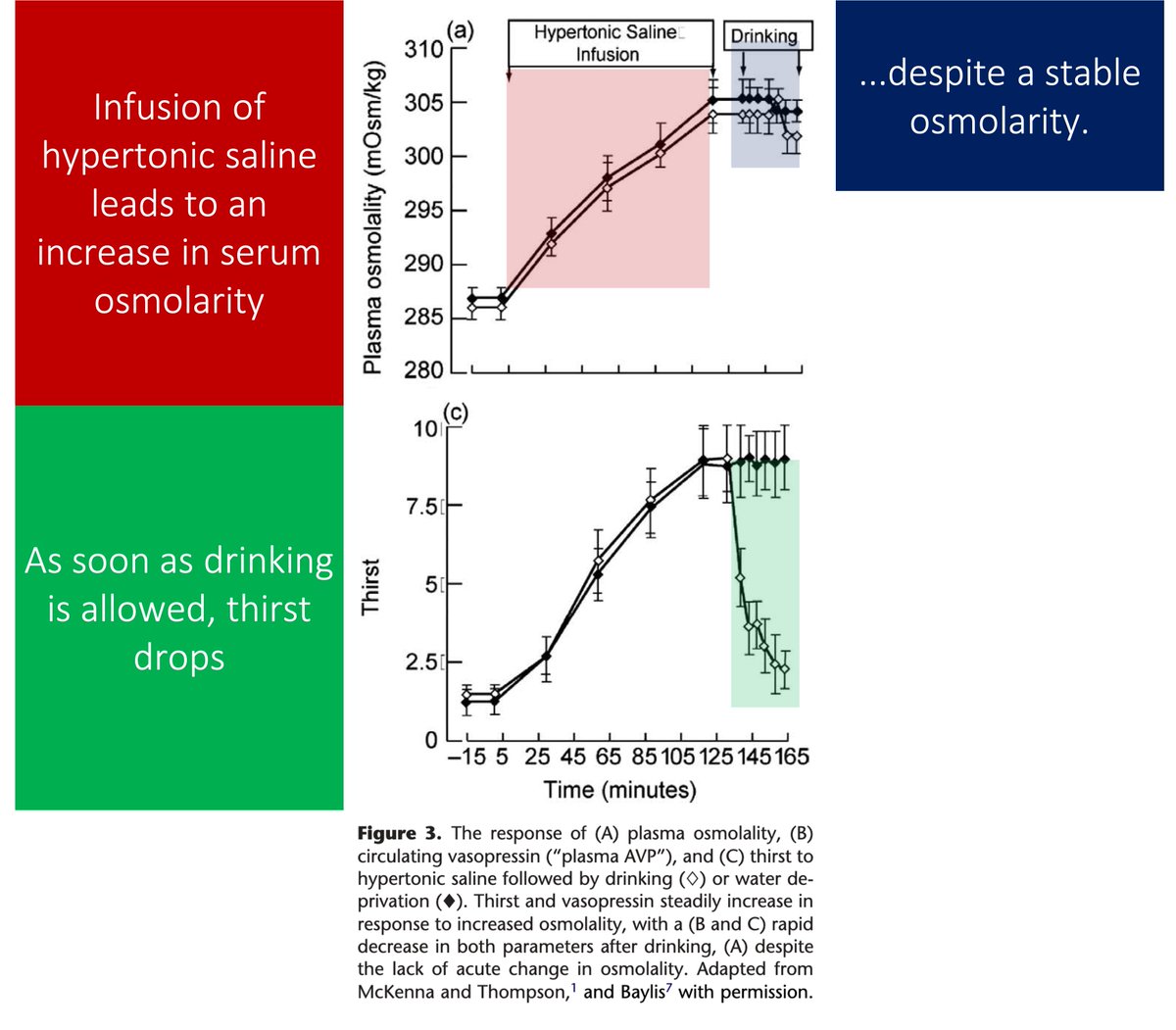
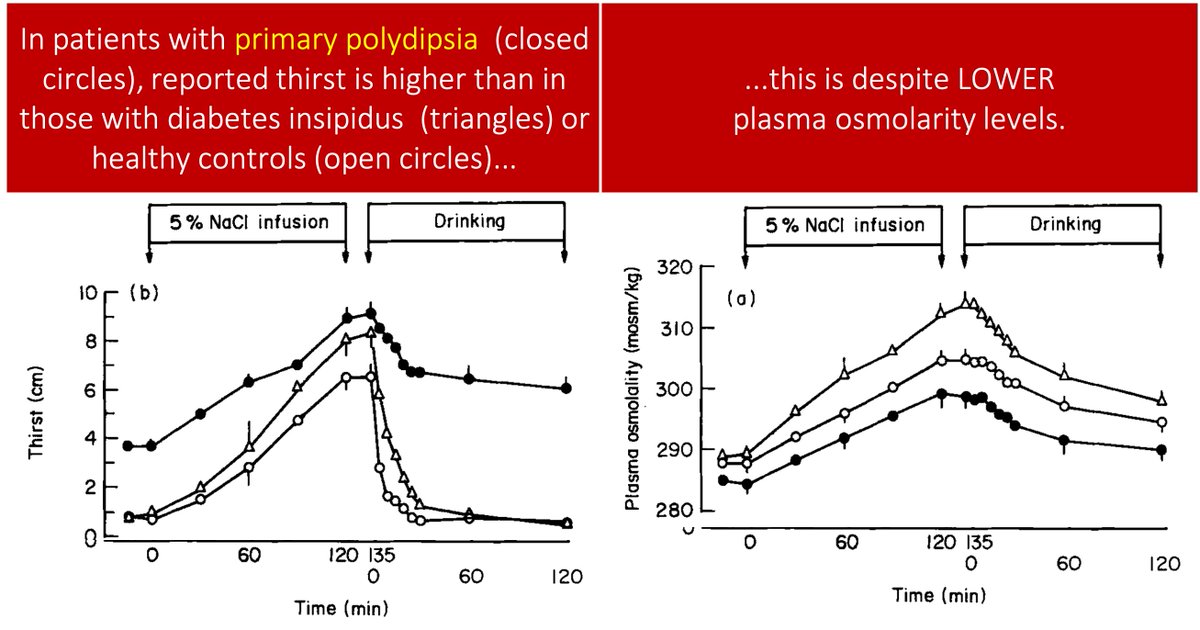
Patients with primary polydipsia report thirst and increased water consumption despite having a LOWER serum osmolarity than those with diabetes insipidus or healthy controls.
And drinking doesn't "quench" the thirst to the same degree.
pubmed.ncbi.nlm.nih.gov/1742879/
Again, patients who become hyponatremic secondary to primary polydipsia continue to drink DESPITE a low osmolarity.
Maybe the osmolarity set point for thirst is lowered.
Or.
Maybe the other driver of thirst plays a role. And, what is the other main driver of thirst?
In response to hypovolemia, angiotensin II (ATII) also acts on the SFO to promote thirst (see the picture in tweet 3).
And, if you inject ATII directly into the SFO, water intake increases.
So, the two drivers of thirst are:
↑Osmolarity
↑ATII
pubmed.ncbi.nlm.nih.gov/9674690/
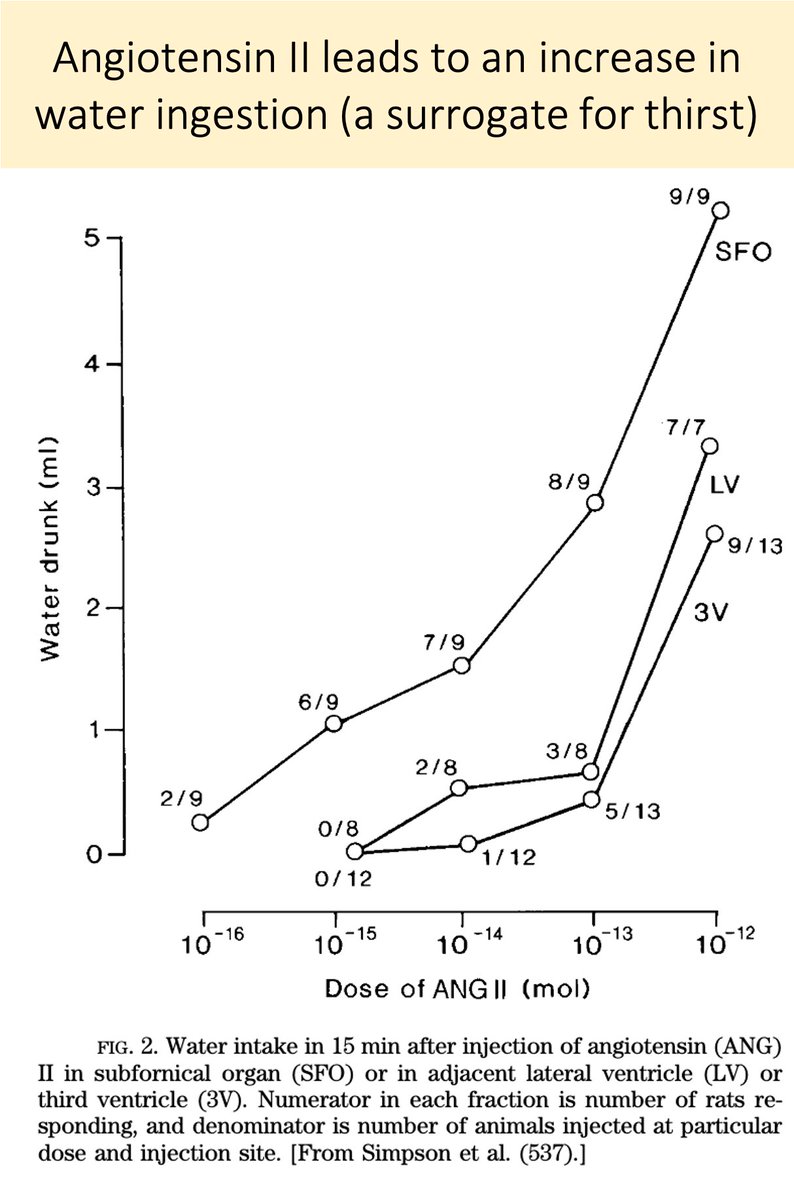
Is ATII involved in the excess drinking seen in primary polydipsia?
Maybe.
To understand how, we must first discuss dopamine.
Animal experiments show that hyperdopaminergic states are associated with increased fluid intake.
And though the mechanism of schizophrenia is clearly multifactorial, the "dopamine hypothesis" remains a leading explanatory model.
pubmed.ncbi.nlm.nih.gov/240934/
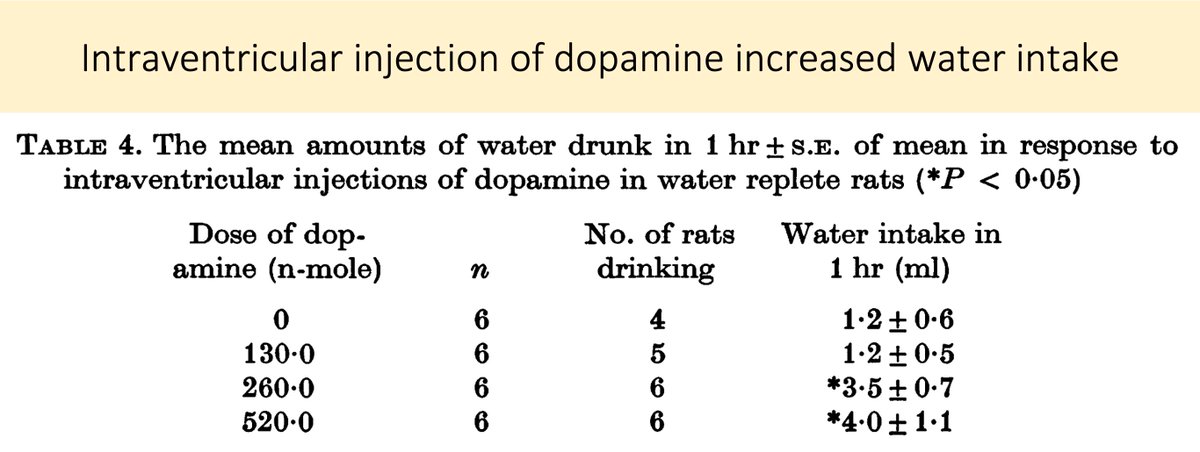
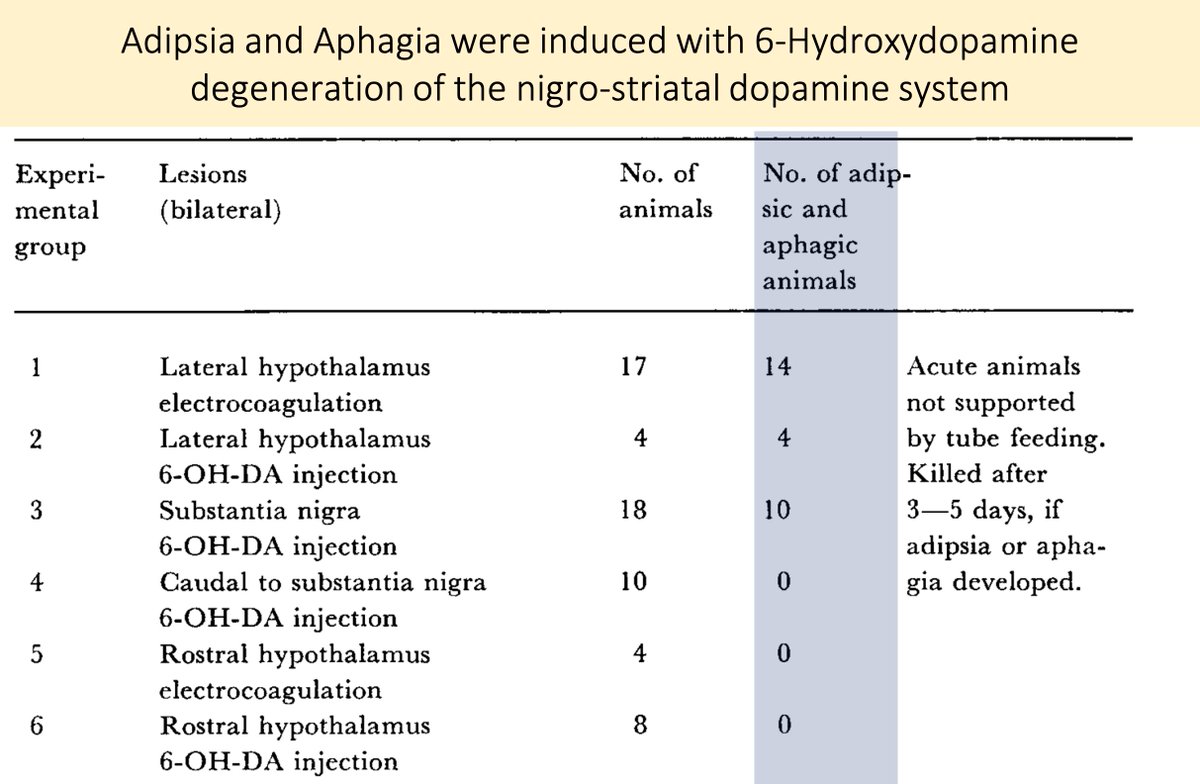
In fact, lesions of the dopaminergic nigrostriatal pathway induced by 6-hydroxy dopamine cause rats to become adipsic (i.e., they stop drinking!)
pubmed.ncbi.nlm.nih.gov/4332694/
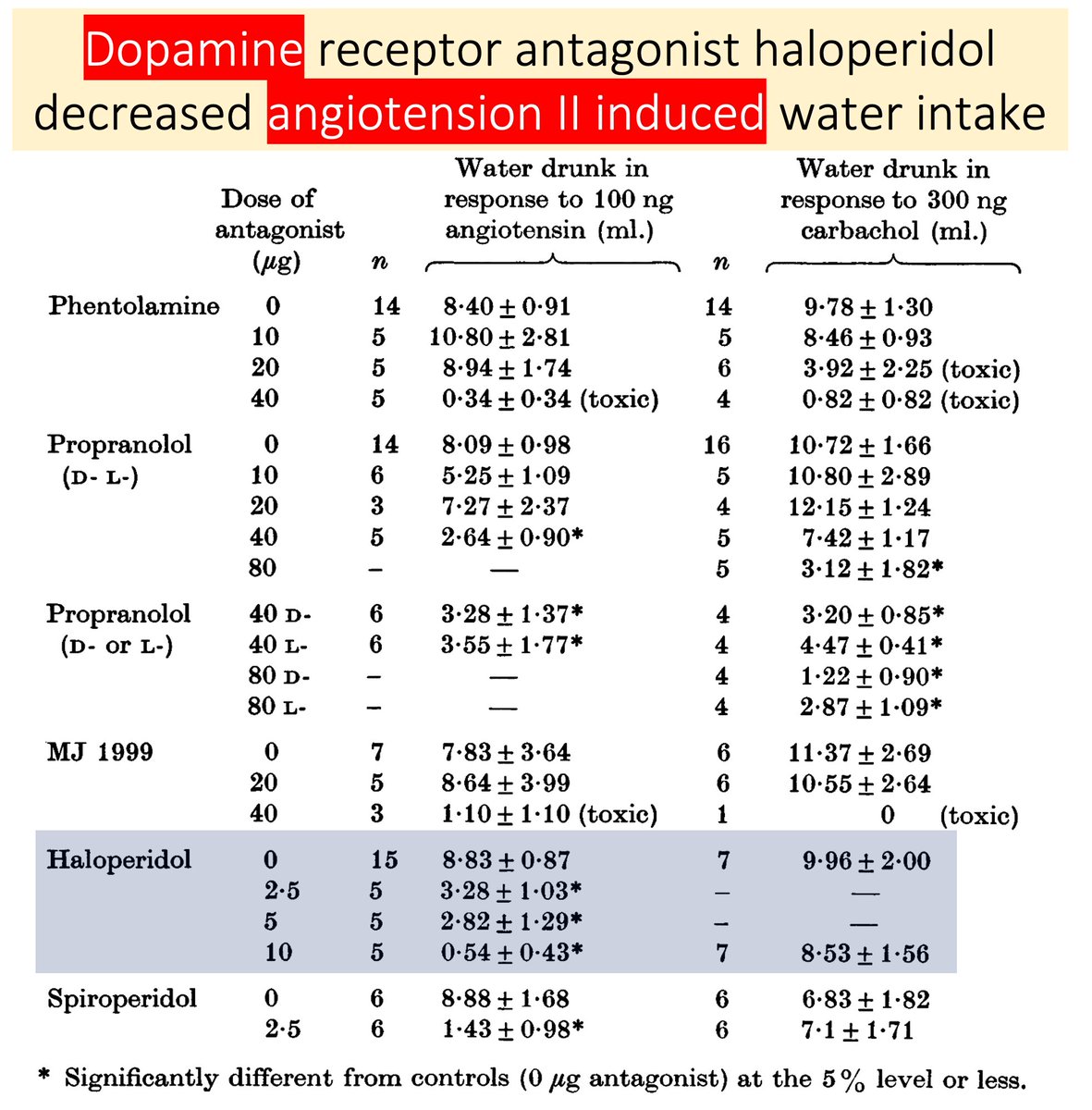
Supporting the role of dopamine is the observation that D2-antagonists (e.g., haloperidol) injected intracranially blocked ATII-induced drinking.
These findings suggest an interplay between dopaminergic systems and ATII-induced thirst.
pubmed.ncbi.nlm.nih.gov/240934/
Interim summary:
◾️ATII promotes thirst
◾️Hyperdopaminergic states are associated with polydipsia
◾️Injection of D2 antagonists blunt ATII-mediated drinking
If patients with primary polydipsia have a dopaminergic state, this may lead to thirst/drinking via ATII pathways.
But: D2-antagonists (eg haloperidol) are associated with an INCREASED risk of polydipsia.
How can this be explained in light of the above?
If acute injection of a D2-antagonist leads to decreased drinking, why would chronic use lead to an increase?
ncbi.nlm.nih.gov/pubmed/31472167
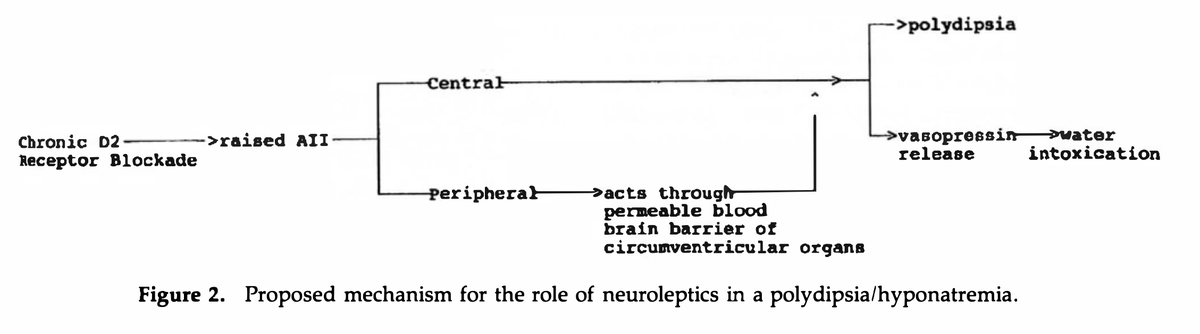
In 1993 Verghese et al proposed the following:
Chronic D2 blockade induced by typical neuroleptics may increase ATII levels, leading to increased thirst, and polydipsia.
pubmed.ncbi.nlm.nih.gov/8105791/
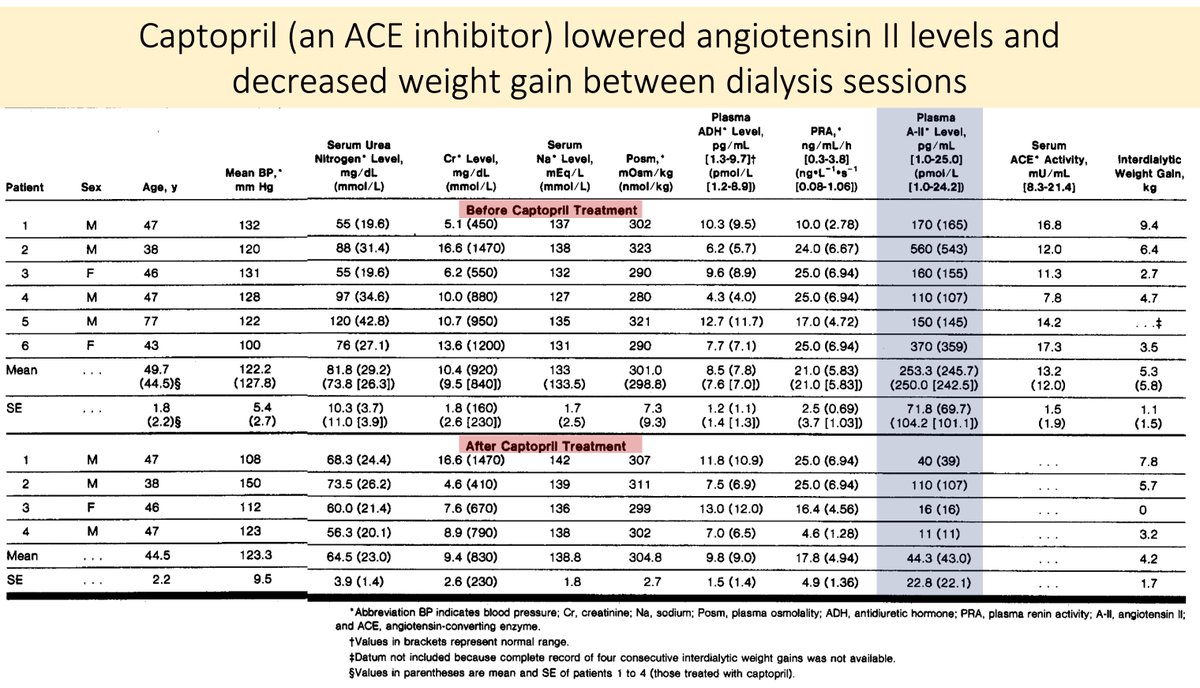
Unsurprisingly, ACE inhibitors have been used in high ATII states associated with increased thirst and water intake.
One example is chronic kidney disease, where some patients have high renin, high ATII, and excessive thirst.
Captopril blunts this.
pubmed.ncbi.nlm.nih.gov/3522948/
The literature I found for the use of ACE inhibitors (and ARBs) in the treatment of primary polydipsia was mostly restricted to case reports.
And, despite the mechanistic underpinning, these results are mixed.
The condition is quite hard to treat.
pubmed.ncbi.nlm.nih.gov/2183881/
💥Increased osmolarity and angiotensin II (ATII) promote thirst
💥In primary polydipsia, osmolarity is low
💥The complex relationship between dopamine, ATII, and thirst may have a role in primary polydipsia